Heterogeneous polymerase fidelity and mismatch repair bias genome variation and composition
- PMID: 25217194
- PMCID: PMC4216917
- DOI: 10.1101/gr.178335.114
Heterogeneous polymerase fidelity and mismatch repair bias genome variation and composition
Abstract
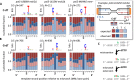
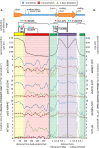
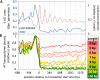
Mutational heterogeneity must be taken into account when reconstructing evolutionary histories, calibrating molecular clocks, and predicting links between genes and disease. Selective pressures and various DNA transactions have been invoked to explain the heterogeneous distribution of genetic variation between species, within populations, and in tissue-specific tumors. To examine relationships between such heterogeneity and variations in leading- and lagging-strand replication fidelity and mismatch repair, we accumulated 40,000 spontaneous mutations in eight diploid yeast strains in the absence of selective pressure. We found that replicase error rates vary by fork direction, coding state, nucleosome proximity, and sequence context. Further, error rates and DNA mismatch repair efficiency both vary by mismatch type, responsible polymerase, replication time, and replication origin proximity. Mutation patterns implicate replication infidelity as one driver of variation in somatic and germline evolution, suggest mechanisms of mutual modulation of genome stability and composition, and predict future observations in specific cancers.
© 2014 Lujan et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Similar articles
-
Ribonucleotides are signals for mismatch repair of leading-strand replication errors.Mol Cell. 2013 May 9;50(3):437-43. doi: 10.1016/j.molcel.2013.03.017. Epub 2013 Apr 18. Mol Cell. 2013. PMID: 23603118 Free PMC article.
-
Evidence that DNA polymerase δ proofreads errors made by DNA polymerase α across the Saccharomyces cerevisiae nuclear genome.DNA Repair (Amst). 2024 Nov;143:103768. doi: 10.1016/j.dnarep.2024.103768. Epub 2024 Sep 21. DNA Repair (Amst). 2024. PMID: 39332392
-
Exonuclease 1 preferentially repairs mismatches generated by DNA polymerase α.DNA Repair (Amst). 2013 Feb 1;12(2):92-6. doi: 10.1016/j.dnarep.2012.11.001. Epub 2012 Dec 11. DNA Repair (Amst). 2013. PMID: 23245696 Free PMC article.
-
Balancing eukaryotic replication asymmetry with replication fidelity.Curr Opin Chem Biol. 2011 Oct;15(5):620-6. doi: 10.1016/j.cbpa.2011.07.025. Epub 2011 Aug 19. Curr Opin Chem Biol. 2011. PMID: 21862387 Free PMC article. Review.
-
Yeast DNA polymerases.Ann N Y Acad Sci. 1992 Oct 13;665:52-8. doi: 10.1111/j.1749-6632.1992.tb42573.x. Ann N Y Acad Sci. 1992. PMID: 1329600 Review. No abstract available.
Cited by
-
Pol32, an accessory subunit of DNA polymerase delta, plays an essential role in genome stability and pathogenesis of Candida albicans.Gut Microbes. 2023 Jan-Dec;15(1):2163840. doi: 10.1080/19490976.2022.2163840. Gut Microbes. 2023. PMID: 36601868 Free PMC article.
-
The absence of the catalytic domains of Saccharomyces cerevisiae DNA polymerase ϵ strongly reduces DNA replication fidelity.Nucleic Acids Res. 2019 May 7;47(8):3986-3995. doi: 10.1093/nar/gkz048. Nucleic Acids Res. 2019. PMID: 30698744 Free PMC article.
-
Mutational signatures of DNA mismatch repair deficiency in C. elegans and human cancers.Genome Res. 2018 May;28(5):666-675. doi: 10.1101/gr.226845.117. Epub 2018 Apr 10. Genome Res. 2018. PMID: 29636374 Free PMC article.
-
Roles for DNA polymerase δ in initiating and terminating leading strand DNA replication.Nat Commun. 2019 Sep 5;10(1):3992. doi: 10.1038/s41467-019-11995-z. Nat Commun. 2019. PMID: 31488849 Free PMC article.
-
High fidelity DNA ligation prevents single base insertions in the yeast genome.Nat Commun. 2024 Oct 9;15(1):8730. doi: 10.1038/s41467-024-53063-1. Nat Commun. 2024. PMID: 39379399 Free PMC article.
References
-
- Agier N, Fischer G. 2012. The mutational profile of the yeast genome is shaped by replication. Mol Biol Evol 29: 905–913 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
