Epigenetic regulation of spinal cord gene expression controls opioid-induced hyperalgesia
- PMID: 25217253
- PMCID: PMC4171542
- DOI: 10.1186/1744-8069-10-59
Epigenetic regulation of spinal cord gene expression controls opioid-induced hyperalgesia
Abstract
Background: The long term use of opioids for the treatment of pain leads to a group of maladaptations which includes opioid-induced hyperalgesia (OIH). OIH typically resolves within few days after cessation of morphine treatment in mice but is prolonged for weeks if histone deacetylase (HDAC) activity is inhibited during opioid treatment. The present work seeks to identify gene targets supporting the epigenetic effects responsible for OIH prolongation.
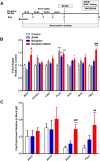
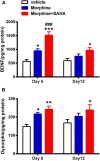
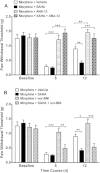
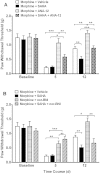
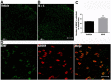
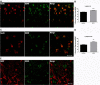
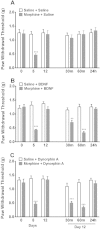
Results: Mice were treated with morphine according to an ascending dose protocol. Some mice also received the selective HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) additionally. Chronic morphine treatment with simultaneous HDAC inhibition enhanced OIH, and several spinal cord genes were up-regulated. The expression of Bdnf (Brain-derived neurotrophic factor) and Pdyn (Prodynorphin) were most closely related to the observed behavioral changes. ChIP (Chromatin immuoprecipation) assays demonstrated that promoter regions of Pdyn and Bdnf were strongly associated with aceH3K9 (Acetylated histone H3 Lysine9) after morphine and SAHA treatment. Furthermore, morphine treatment caused an increase in spinal BDNF and dynorphin levels, and these levels were further increased in SAHA treated mice. The selective TrkB (tropomyosin-receptor-kinase) antagonist ANA-12 reduced OIH when given one or seven days after cessation of morphine. Treatment with the selective kappa opioid receptor antagonist nor-BNI also reduced established OIH. The co-administration of either receptor antagonist agent daily with morphine resulted in attenuation of hyperalgesia present one day after cessation of treatment. Additionally, repeated morphine exposure induced a rise in BDNF expression that was associated with an increased number of BDNF+ cells in the spinal cord dorsal horn, showing strong co-localization with aceH3K9 in neuronal cells. Lastly, spinal application of low dose BDNF or Dynorphin A after resolution of OIH produced mechanical hypersensitivity, with no effect in controls.
Conclusions: The present study identified two genes whose expression is regulated by epigenetic mechanisms during morphine exposure. Treatments aimed at preventing the acetylation of histones or blocking BDNF and dynorphin signaling may reduce OIH and improve long-term pain using opioids.
Figures
Similar articles
-
Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure.Mol Pain. 2016 Apr 18;12:1744806916641950. doi: 10.1177/1744806916641950. Print 2016. Mol Pain. 2016. PMID: 27094549 Free PMC article.
-
Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice.J Pain. 2013 Jan;14(1):36-47. doi: 10.1016/j.jpain.2012.10.005. J Pain. 2013. PMID: 23273833 Free PMC article.
-
Demethylation regulation of BDNF gene expression in dorsal root ganglion neurons is implicated in opioid-induced pain hypersensitivity in rats.Neurochem Int. 2016 Jul;97:91-8. doi: 10.1016/j.neuint.2016.03.007. Epub 2016 Mar 10. Neurochem Int. 2016. PMID: 26970395
-
Opioid tolerance and opioid-induced hyperalgesia: Is TrkB modulation a potential pharmacological solution?Neuropharmacology. 2022 Dec 1;220:109260. doi: 10.1016/j.neuropharm.2022.109260. Epub 2022 Sep 19. Neuropharmacology. 2022. PMID: 36165856 Review.
-
Opioid induced hyperalgesia: clinical implications for the pain practitioner.Pain Physician. 2009 May-Jun;12(3):679-84. Pain Physician. 2009. PMID: 19461836 Review.
Cited by
-
Epigenetic regulation in opioid induced hyperalgesia.Neurobiol Pain. 2023 Nov 23;14:100146. doi: 10.1016/j.ynpai.2023.100146. eCollection 2023 Aug-Dec. Neurobiol Pain. 2023. PMID: 38099284 Free PMC article. Review.
-
A review of the kappa opioid receptor system in opioid use.Neurosci Biobehav Rev. 2024 Jul;162:105713. doi: 10.1016/j.neubiorev.2024.105713. Epub 2024 May 10. Neurosci Biobehav Rev. 2024. PMID: 38733895 Free PMC article.
-
Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure.Mol Pain. 2016 Apr 18;12:1744806916641950. doi: 10.1177/1744806916641950. Print 2016. Mol Pain. 2016. PMID: 27094549 Free PMC article.
-
Chronic Pain and Psychiatric Conditions.Complex Psychiatry. 2022 Sep 15;9(1-4):24-43. doi: 10.1159/000527041. eCollection 2023 Jan-Dec. Complex Psychiatry. 2022. PMID: 37034825 Free PMC article. Review.
-
The role of the gut microbiome and microbial metabolism in mediating opioid-induced changes in the epigenome.Front Microbiol. 2023 Aug 21;14:1233194. doi: 10.3389/fmicb.2023.1233194. eCollection 2023. Front Microbiol. 2023. PMID: 37670983 Free PMC article. Review.
References
-
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. - PubMed
-
- Chapman CR, Lipschitz DL, Angst MS, Chou R, Denisco RC, Donaldson GW, Fine PG, Foley KM, Gallagher RM, Gilson AM, Haddox JD, Horn SD, Inturrisi CE, Jick SS, Lipman AG, Loeser JD, Noble M, Porter L, Rowbotham MC, Schoelles KM, Turk DC, Volinn E, Von Korff MR, Webster LR, Weisner CM. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain. 2010;11(9):807–829. doi: 10.1016/j.jpain.2010.02.019. - DOI - PubMed
-
- Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician. 2011;14(2):E133–E156. - PubMed
-
- Paulozzi L, Baldwin G, Franklin G, Gil Kerlikowske R, Jones CM, Ghiya N. CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

