Enhanced glucose tolerance in pancreatic-derived factor (PANDER) knockout C57BL/6 mice
- PMID: 25217499
- PMCID: PMC4213734
- DOI: 10.1242/dmm.016402
Enhanced glucose tolerance in pancreatic-derived factor (PANDER) knockout C57BL/6 mice
Abstract
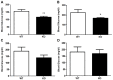
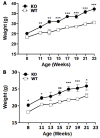
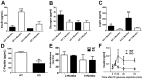
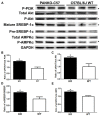
Pancreatic-derived factor (PANDER; also known as FAM3B) is a uniquely structured protein strongly expressed within and secreted from the endocrine pancreas. PANDER has been hypothesized to regulate fasting and fed glucose homeostasis, hepatic lipogenesis and insulin signaling, and to serve a potential role in the onset or progression of type 2 diabetes (T2D). Despite having potentially pivotal pleiotropic roles in glycemic regulation and T2D, there has been limited generation of stable animal models for the investigation of PANDER function, and there are no models on well-established genetic murine backgrounds for T2D. Our aim was to generate an enhanced murine model to further elucidate the biological function of PANDER. Therefore, a pure-bred PANDER knockout C57BL/6 (PANKO-C57) model was created and phenotypically characterized with respect to glycemic regulation and hepatic insulin signaling. The PANKO-C57 model exhibited an enhanced metabolic phenotype, particularly with regard to enhanced glucose tolerance. Male PANKO-C57 mice displayed decreased fasting plasma insulin and C-peptide levels, whereas leptin levels were increased as compared with matched C57BL/6J wild-type mice. Despite similar peripheral insulin sensitivity between both groups, hepatic insulin signaling was significantly increased during fasting conditions, as demonstrated by increased phosphorylation of hepatic PKB/Akt and AMPK, along with mature SREBP-1 expression. Insulin stimulation of PANKO-C57 mice resulted in increased hepatic triglyceride and glycogen content as compared with wild-type C57BL/6 mice. In summary, the PANKO-C57 mouse represents a suitable model for the investigation of PANDER in multiple metabolic states and provides an additional tool to elucidate the biological function and potential role in T2D.
Keywords: FAM3B; Glucose tolerance; Glycemic regulation; Hepatic insulin signaling; Knockout model; Pancreatic-derived factor.
© 2014. Published by The Company of Biologists Ltd.
Figures


Similar articles
-
PANDER transgenic mice display fasting hyperglycemia and hepatic insulin resistance.J Endocrinol. 2014 Jan 27;220(3):219-31. doi: 10.1530/JOE-13-0338. Print 2014 Mar. J Endocrinol. 2014. PMID: 24468680
-
Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function.Diabetes. 2010 Sep;59(9):2209-18. doi: 10.2337/db09-1552. Epub 2010 Jun 21. Diabetes. 2010. PMID: 20566664 Free PMC article.
-
Liver-specific overexpression of pancreatic-derived factor (PANDER) induces fasting hyperglycemia in mice.Endocrinology. 2010 Nov;151(11):5174-84. doi: 10.1210/en.2010-0379. Epub 2010 Sep 15. Endocrinology. 2010. PMID: 20844005 Free PMC article.
-
PANcreatic-DERived factor: novel hormone PANDERing to glucose regulation.FEBS Lett. 2011 Jul 21;585(14):2137-43. doi: 10.1016/j.febslet.2011.05.059. Epub 2011 Jun 12. FEBS Lett. 2011. PMID: 21664909 Free PMC article. Review.
-
Role of pancreatic-derived factor in type 2 diabetes: evidence from pancreatic β cells and liver.Nutr Rev. 2012 Feb;70(2):100-6. doi: 10.1111/j.1753-4887.2011.00457.x. Nutr Rev. 2012. PMID: 22300596 Review.
Cited by
-
Circulating PANDER concentration is associated with increased HbA1c and fasting blood glucose in Type 2 diabetic subjects.J Clin Transl Endocrinol. 2018 Feb 23;11:26-30. doi: 10.1016/j.jcte.2018.02.003. eCollection 2018 Mar. J Clin Transl Endocrinol. 2018. PMID: 29686968 Free PMC article.
-
Identifying potential therapeutic targets of Tang-Yi-Ping for the treatment of impaired glucose tolerance: a tandem mass tag-labeled quantitative proteomic analysis.Ann Transl Med. 2021 Oct;9(20):1532. doi: 10.21037/atm-21-4257. Ann Transl Med. 2021. PMID: 34790738 Free PMC article.
-
A genomics approach reveals insights into the importance of gene losses for mammalian adaptations.Nat Commun. 2018 Mar 23;9(1):1215. doi: 10.1038/s41467-018-03667-1. Nat Commun. 2018. PMID: 29572503 Free PMC article.
-
FAM3C activates HSF1 to suppress hepatic gluconeogenesis and attenuate hyperglycemia of type 1 diabetic mice.Oncotarget. 2017 Nov 20;8(62):106038-106049. doi: 10.18632/oncotarget.22524. eCollection 2017 Dec 1. Oncotarget. 2017. PMID: 29285313 Free PMC article.
-
The cytokine FAM3B/PANDER is an FGFR ligand that promotes posterior development in Xenopus.Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2100342118. doi: 10.1073/pnas.2100342118. Proc Natl Acad Sci U S A. 2021. PMID: 33975953 Free PMC article.
References
-
- Ahima R. S., Flier J. S. (2000). Leptin. Annu. Rev. Physiol. 62, 413–437 - PubMed
-
- Biddinger S. B., Hernandez-Ono A., Rask-Madsen C., Haas J. T., Alemán J. O., Suzuki R., Scapa E. F., Agarwal C., Carey M. C., Stephanopoulos G., et al. (2008). Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 7, 125–134 - PMC - PubMed
-
- Brown M. S., Goldstein J. L. (2008). Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

