HIV-1 DNA predicts disease progression and post-treatment virological control
- PMID: 25217531
- PMCID: PMC4199415
- DOI: 10.7554/eLife.03821
HIV-1 DNA predicts disease progression and post-treatment virological control
Abstract
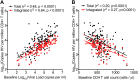
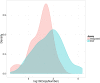
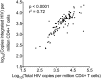
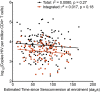
In HIV-1 infection, a population of latently infected cells facilitates viral persistence despite antiretroviral therapy (ART). With the aim of identifying individuals in whom ART might induce a period of viraemic control on stopping therapy, we hypothesised that quantification of the pool of latently infected cells in primary HIV-1 infection (PHI) would predict clinical progression and viral replication following ART. We measured HIV-1 DNA in a highly characterised randomised population of individuals with PHI. We explored associations between HIV-1 DNA and immunological and virological markers of clinical progression, including viral rebound in those interrupting therapy. In multivariable analyses, HIV-1 DNA was more predictive of disease progression than plasma viral load and, at treatment interruption, predicted time to plasma virus rebound. HIV-1 DNA may help identify individuals who could safely interrupt ART in future HIV-1 eradication trials.
Keywords: HIV-1; antiretroviral therapy; cure; human; human biology; infectious disease; medicine; microbiology; primary infection; reservoir.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
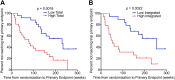
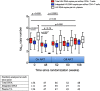
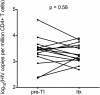
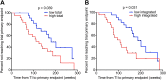
Comment in
-
Biomarker reveals HIV's hidden reservoir.Elife. 2014 Oct 16;3:e04742. doi: 10.7554/eLife.04742. Elife. 2014. PMID: 25321627 Free PMC article.
References
-
- Avettand-Fenoel V, Chaix ML, Blanche S, Burgard M, Floch C, Toure K, Allemon MC, Warszawski J, Rouzioux C, French Pediatric Cohort Study ANRS-CO 01 Group LTR real-time PCR for HIV-1 DNA quantitation in blood cells for early diagnosis in infants born to seropositive mothers treated in HAART area (ANRS CO 01) Journal of Medical Virology. 2009;81:217–223. doi: 10.1002/jmv.21390. - DOI - PubMed
-
- Chun TW, Justement JS, Moir S, Hallahan CW, Maenza J, Mullins JI, Collier AC, Corey L, Fauci AS. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. The Journal of Infectious Diseases. 2007;195:1762–1764. doi: 10.1086/518250. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

