ILDR1 null mice, a model of human deafness DFNB42, show structural aberrations of tricellular tight junctions and degeneration of auditory hair cells
- PMID: 25217574
- PMCID: PMC4291242
- DOI: 10.1093/hmg/ddu474
ILDR1 null mice, a model of human deafness DFNB42, show structural aberrations of tricellular tight junctions and degeneration of auditory hair cells
Abstract
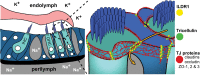
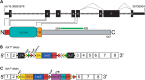
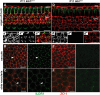
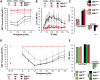
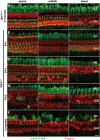
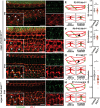
In the mammalian inner ear, bicellular and tricellular tight junctions (tTJs) seal the paracellular space between epithelial cells. Tricellulin and immunoglobulin-like (Ig-like) domain containing receptor 1 (ILDR1, also referred to as angulin-2) localize to tTJs of the sensory and non-sensory epithelia in the organ of Corti and vestibular end organs. Recessive mutations of TRIC (DFNB49) encoding tricellulin and ILDR1 (DFNB42) cause human nonsyndromic deafness. However, the pathophysiology of DFNB42 deafness remains unknown. ILDR1 was recently reported to be a lipoprotein receptor mediating the secretion of the fat-stimulated cholecystokinin (CCK) hormone in the small intestine, while ILDR1 in EpH4 mouse mammary epithelial cells in vitro was shown to recruit tricellulin to tTJs. Here we show that two different mouse Ildr1 mutant alleles have early-onset severe deafness associated with a rapid degeneration of cochlear hair cells (HCs) but have a normal endocochlear potential. ILDR1 is not required for recruitment of tricellulin to tTJs in the cochlea in vivo; however, tricellulin becomes mislocalized in the inner ear sensory epithelia of ILDR1 null mice after the first postnatal week. As revealed by freeze-fracture electron microscopy, ILDR1 contributes to the ultrastructure of inner ear tTJs. Taken together, our data provide insight into the pathophysiology of human DFNB42 deafness and demonstrate that ILDR1 is crucial for normal hearing by maintaining the structural and functional integrity of tTJs, which are critical for the survival of auditory neurosensory HCs.
© The Author 2014. Published by Oxford University Press.
Figures


Similar articles
-
Deficiency of angulin-2/ILDR1, a tricellular tight junction-associated membrane protein, causes deafness with cochlear hair cell degeneration in mice.PLoS One. 2015 Mar 30;10(3):e0120674. doi: 10.1371/journal.pone.0120674. eCollection 2015. PLoS One. 2015. PMID: 25822906 Free PMC article.
-
Analysis of the 'angulin' proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis.J Cell Sci. 2013 Feb 15;126(Pt 4):966-77. doi: 10.1242/jcs.116442. Epub 2012 Dec 13. J Cell Sci. 2013. PMID: 23239027
-
Downsloping high-frequency hearing loss due to inner ear tricellular tight junction disruption by a novel ILDR1 mutation in the Ig-like domain.PLoS One. 2015 Feb 10;10(2):e0116931. doi: 10.1371/journal.pone.0116931. eCollection 2015. PLoS One. 2015. PMID: 25668204 Free PMC article.
-
[Molecular organization of tricellular tight junctions].Yakugaku Zasshi. 2014;134(5):615-21. doi: 10.1248/yakushi.14-00006-1. Yakugaku Zasshi. 2014. PMID: 24790043 Review. Japanese.
-
Tricellular Tight Junctions in the Inner Ear.Biomed Res Int. 2016;2016:6137541. doi: 10.1155/2016/6137541. Epub 2016 Apr 18. Biomed Res Int. 2016. PMID: 27195292 Free PMC article. Review.
Cited by
-
Copy number variation in the MSRB3 gene enlarges porcine ear size through a mechanism involving miR-584-5p.Genet Sel Evol. 2018 Dec 27;50(1):72. doi: 10.1186/s12711-018-0442-6. Genet Sel Evol. 2018. PMID: 30587124 Free PMC article.
-
ILDR1 deficiency causes degeneration of cochlear outer hair cells and disrupts the structure of the organ of Corti: a mouse model for human DFNB42.Biol Open. 2015 Mar 27;4(4):411-8. doi: 10.1242/bio.201410876. Biol Open. 2015. PMID: 25819842 Free PMC article.
-
The phenotypic landscape of a Tbc1d24 mutant mouse includes convulsive seizures resembling human early infantile epileptic encephalopathy.Hum Mol Genet. 2019 May 1;28(9):1530-1547. doi: 10.1093/hmg/ddy445. Hum Mol Genet. 2019. PMID: 30602030 Free PMC article.
-
Mouse Models of Human Pathogenic Variants of TBC1D24 Associated with Non-Syndromic Deafness DFNB86 and DFNA65 and Syndromes Involving Deafness.Genes (Basel). 2020 Sep 24;11(10):1122. doi: 10.3390/genes11101122. Genes (Basel). 2020. PMID: 32987832 Free PMC article.
-
Deletion of Brg1 causes abnormal hair cell planer polarity, hair cell anchorage, and scar formation in mouse cochlea.Sci Rep. 2016 Jun 3;6:27124. doi: 10.1038/srep27124. Sci Rep. 2016. PMID: 27255603 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 DK091946/DK/NIDDK NIH HHS/United States
- WT100669MA/WT_/Wellcome Trust/United Kingdom
- DK091946/DK/NIDDK NIH HHS/United States
- R01 DC012151/DC/NIDCD NIH HHS/United States
- DC000039-18/DC/NIDCD NIH HHS/United States
- I01 BX002230/BX/BLRD VA/United States
- DC000060-14/DC/NIDCD NIH HHS/United States
- 100669/WT_/Wellcome Trust/United Kingdom
- R01-DC012151/DC/NIDCD NIH HHS/United States
- G0300212/MRC_/Medical Research Council/United Kingdom
- R01 DK098796/DK/NIDDK NIH HHS/United States
- MC_QA137918/MRC_/Medical Research Council/United Kingdom
- DK098796/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

