Co-activator independent differences in how the metaphase and anaphase APC/C recognise the same substrate
- PMID: 25217616
- PMCID: PMC4197439
- DOI: 10.1242/bio.20149415
Co-activator independent differences in how the metaphase and anaphase APC/C recognise the same substrate
Abstract
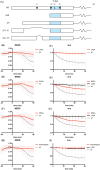
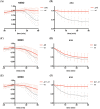
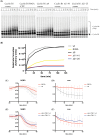
The Anaphase Promoting Complex or Cyclosome (APC/C) is critical to the control of mitosis. The APC/C is an ubiquitin ligase that targets specific mitotic regulators for proteolysis at distinct times in mitosis, but how this is achieved is not well understood. We have addressed this question by determining whether the same substrate, cyclin B1, is recognised in the same way by the APC/C at different times in mitosis. Unexpectedly, we find that distinct but overlapping motifs in cyclin B1 are recognised by the APC/C in metaphase compared with anaphase, and this does not depend on the exchange of Cdc20 for Cdh1. Thus, changes in APC/C substrate specificity in mitosis can potentially be conferred by altering interaction sites in addition to exchanging Cdc20 for Cdh1.
Keywords: Anaphase Promoting Complex/Cyclosome; Cyclin B; Mitosis.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures

Similar articles
-
The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis.Curr Biol. 1998 Jun 18;8(13):750-60. doi: 10.1016/s0960-9822(98)70298-2. Curr Biol. 1998. PMID: 9651679
-
How APC/C-Cdc20 changes its substrate specificity in mitosis.Nat Cell Biol. 2011 Mar;13(3):223-33. doi: 10.1038/ncb2165. Epub 2011 Feb 20. Nat Cell Biol. 2011. PMID: 21336306 Free PMC article.
-
The HECT type ubiquitin ligase NEDL2 is degraded by anaphase-promoting complex/cyclosome (APC/C)-Cdh1, and its tight regulation maintains the metaphase to anaphase transition.J Biol Chem. 2013 Dec 13;288(50):35637-50. doi: 10.1074/jbc.M113.472076. Epub 2013 Oct 25. J Biol Chem. 2013. PMID: 24163370 Free PMC article.
-
Mitotic regulation of the anaphase-promoting complex.Cell Mol Life Sci. 2007 Mar;64(5):589-600. doi: 10.1007/s00018-007-6443-1. Cell Mol Life Sci. 2007. PMID: 17334950 Free PMC article. Review.
-
Control of mitotic transitions by the anaphase-promoting complex.Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1583-90. doi: 10.1098/rstb.1999.0502. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582244 Free PMC article. Review.
Cited by
-
Spatiotemporal regulation of the anaphase-promoting complex in mitosis.Nat Rev Mol Cell Biol. 2015 Feb;16(2):82-94. doi: 10.1038/nrm3934. Nat Rev Mol Cell Biol. 2015. PMID: 25604195 Free PMC article. Review.
-
APC/CFZR-1 regulates centrosomal ZYG-1 to limit centrosome number.J Cell Sci. 2021 Jul 15;134(14):jcs253088. doi: 10.1242/jcs.253088. Epub 2021 Jul 26. J Cell Sci. 2021. PMID: 34308970 Free PMC article.
-
Interphase APC/C-Cdc20 inhibition by cyclin A2-Cdk2 ensures efficient mitotic entry.Nat Commun. 2016 Mar 10;7:10975. doi: 10.1038/ncomms10975. Nat Commun. 2016. PMID: 26960431 Free PMC article.
-
Spatial control of the APC/C ensures the rapid degradation of cyclin B1.EMBO J. 2024 Oct;43(19):4324-4355. doi: 10.1038/s44318-024-00194-2. Epub 2024 Aug 14. EMBO J. 2024. PMID: 39143240 Free PMC article.
-
Spatiotemporal control of mitotic exit during anaphase by an aurora B-Cdk1 crosstalk.Elife. 2019 Aug 19;8:e47646. doi: 10.7554/eLife.47646. Elife. 2019. PMID: 31424385 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

