Phosphoinositide 3-kinase p85beta regulates invadopodium formation
- PMID: 25217619
- PMCID: PMC4197441
- DOI: 10.1242/bio.20148185
Phosphoinositide 3-kinase p85beta regulates invadopodium formation
Abstract
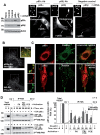
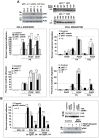
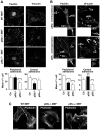
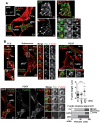
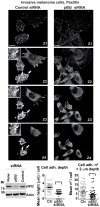
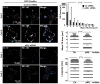
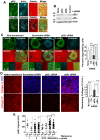
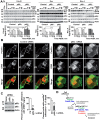
The acquisition of invasiveness is characteristic of tumor progression. Numerous genetic changes are associated with metastasis, but the mechanism by which a cell becomes invasive remains unclear. Expression of p85β, a regulatory subunit of phosphoinositide-3-kinase, markedly increases in advanced carcinoma, but its mode of action is unknown. We postulated that p85β might facilitate cell invasion. We show that p85β localized at cell adhesions in complex with focal adhesion kinase and enhanced stability and maturation of cell adhesions. In addition, p85β induced development at cell adhesions of an F-actin core that extended several microns into the cell z-axis resembling the skeleton of invadopodia. p85β lead to F-actin polymerization at cell adhesions by recruiting active Cdc42/Rac at these structures. In accordance with p85β function in invadopodium-like formation, p85β levels increased in metastatic melanoma and p85β depletion reduced invadopodium formation and invasion. These results show that p85β enhances invasion by inducing cell adhesion development into invadopodia-like structures explaining the metastatic potential of tumors with increased p85β levels.
Keywords: cell adhesion; invadopodium; invasion; metastasis; p85β.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures
References
-
- Bartolomé R. A., Molina-Ortiz I., Samaniego R., Sánchez-Mateos P., Bustelo X. R., Teixidó J. (2006). Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 66, 248–258 10.1158/0008-5472.CAN-05-2489 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

