Novel roles for actin in mitochondrial fission
- PMID: 25217628
- PMCID: PMC4215709
- DOI: 10.1242/jcs.153791
Novel roles for actin in mitochondrial fission
Abstract
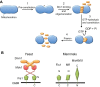
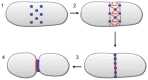
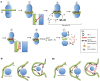
Mitochondrial dynamics, including fusion, fission and translocation, are crucial to cellular homeostasis, with roles in cellular polarity, stress response and apoptosis. Mitochondrial fission has received particular attention, owing to links with several neurodegenerative diseases. A central player in fission is the cytoplasmic dynamin-related GTPase Drp1, which oligomerizes at the fission site and hydrolyzes GTP to drive membrane ingression. Drp1 recruitment to the outer mitochondrial membrane (OMM) is a key regulatory event, which appears to require a pre-constriction step in which the endoplasmic reticulum (ER) and mitochondrion interact extensively, a process termed ERMD (ER-associated mitochondrial division). It is unclear how ER-mitochondrial contact generates the force required for pre-constriction or why pre-constriction leads to Drp1 recruitment. Recent results, however, show that ERMD might be an actin-based process in mammals that requires the ER-associated formin INF2 upstream of Drp1, and that myosin II and other actin-binding proteins might be involved. In this Commentary, we present a mechanistic model for mitochondrial fission in which actin and myosin contribute in two ways; firstly, by supplying the force for pre-constriction and secondly, by serving as a coincidence detector for Drp1 binding. In addition, we discuss the possibility that multiple fission mechanisms exist in mammals.
Keywords: Actin; Mitochondrial fission; Myosin.
© 2014. Published by The Company of Biologists Ltd.
Figures

Similar articles
-
An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2.Science. 2013 Jan 25;339(6118):464-7. doi: 10.1126/science.1228360. Science. 2013. PMID: 23349293 Free PMC article.
-
A role for myosin II in mammalian mitochondrial fission.Curr Biol. 2014 Feb 17;24(4):409-14. doi: 10.1016/j.cub.2013.12.032. Epub 2014 Jan 30. Curr Biol. 2014. PMID: 24485837 Free PMC article.
-
Mitochondria- and ER-associated actin are required for mitochondrial fusion.Nat Commun. 2025 Jan 7;16(1):451. doi: 10.1038/s41467-024-55758-x. Nat Commun. 2025. PMID: 39774009 Free PMC article.
-
The Drp1-Mediated Mitochondrial Fission Protein Interactome as an Emerging Core Player in Mitochondrial Dynamics and Cardiovascular Disease Therapy.Int J Mol Sci. 2023 Mar 17;24(6):5785. doi: 10.3390/ijms24065785. Int J Mol Sci. 2023. PMID: 36982862 Free PMC article. Review.
-
The Role of Mitochondrial Dynamics and Mitotic Fission in Regulating the Cell Cycle in Cancer and Pulmonary Arterial Hypertension: Implications for Dynamin-Related Protein 1 and Mitofusin2 in Hyperproliferative Diseases.Cells. 2023 Jul 20;12(14):1897. doi: 10.3390/cells12141897. Cells. 2023. PMID: 37508561 Free PMC article. Review.
Cited by
-
Enzymatic Noncovalent Synthesis.Chem Rev. 2020 Sep 23;120(18):9994-10078. doi: 10.1021/acs.chemrev.0c00306. Epub 2020 Aug 19. Chem Rev. 2020. PMID: 32812754 Free PMC article. Review.
-
Mitochondrial cellular organization and shape fluctuations are differentially modulated by cytoskeletal networks.Sci Rep. 2023 Mar 11;13(1):4065. doi: 10.1038/s41598-023-31121-w. Sci Rep. 2023. PMID: 36906690 Free PMC article.
-
Suppression of colorectal cancer cell growth by combined treatment of 6-gingerol and γ-tocotrienol via alteration of multiple signalling pathways.J Nat Med. 2019 Sep;73(4):745-760. doi: 10.1007/s11418-019-01323-6. Epub 2019 Jun 8. J Nat Med. 2019. PMID: 31177355
-
Essential role for paxillin tyrosine phosphorylation in LPS-induced mitochondrial fission, ROS generation and lung endothelial barrier loss.Sci Rep. 2021 Sep 2;11(1):17546. doi: 10.1038/s41598-021-97006-y. Sci Rep. 2021. PMID: 34475475 Free PMC article.
-
Abnormalities of Mitochondrial Dynamics in Neurodegenerative Diseases.Antioxidants (Basel). 2017 Apr 5;6(2):25. doi: 10.3390/antiox6020025. Antioxidants (Basel). 2017. PMID: 28379197 Free PMC article. Review.
References
-
- Benda C. (1898). Arch. Anat. Physiol. 73, 393–398
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

