A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration
- PMID: 25217632
- PMCID: PMC6518324
- DOI: 10.1242/jcs.155861
A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration
Abstract
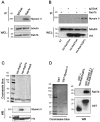
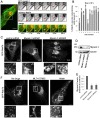
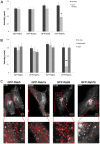
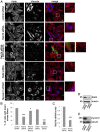
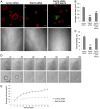
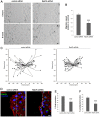
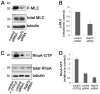
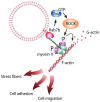
Rab proteins are small GTPases that regulate transport between the different compartments of the endomembrane system in eukaryotic cells. Here, we show that Rab7b, a Rab that controls the transport between late endosomes and the trans Golgi network, interacts directly with myosin II. We illustrate the functional relevance of this interaction, demonstrating that myosin II mediates the transport of Rab7b endosomes, as Rab7b dynamics are strongly affected after myosin II depletion or inhibition. We also demonstrate that a member of the Rab family regulates actin remodeling and, consequently, influences cell adhesion, polarization and migration. We find the molecular mechanism by which Rab7b influences stress fiber formation - through controlling the activation status of the small GTPase RhoA and therefore influencing myosin light chain phosphorylation. Our findings reveal a newly identified role for Rab proteins outside of their canonical role in intracellular trafficking, identifying Rab7b as a coordinator of cytoskeletal organization.
Keywords: Actomyosin; Endosomes; Rab proteins; Rab7b.
© 2014. Published by The Company of Biologists Ltd.
Figures
References
-
- Allaire P. D., Seyed Sadr M., Chaineau M., Seyed Sadr E., Konefal S., Fotouhi M., Maret D., Ritter B., Del Maestro R. F., McPherson P. S. (2013). Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J. Cell Sci. 126, 722–731. 10.1242/jcs.112375 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

