Interaction of the molecular chaperone DNAJB6 with growing amyloid-beta 42 (Aβ42) aggregates leads to sub-stoichiometric inhibition of amyloid formation
- PMID: 25217638
- PMCID: PMC4223311
- DOI: 10.1074/jbc.M114.595124
Interaction of the molecular chaperone DNAJB6 with growing amyloid-beta 42 (Aβ42) aggregates leads to sub-stoichiometric inhibition of amyloid formation
Abstract
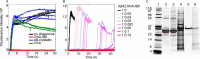
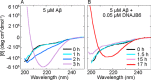
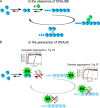
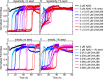
The human molecular chaperone protein DNAJB6 was recently found to inhibit the formation of amyloid fibrils from polyglutamine peptides associated with neurodegenerative disorders such as Huntington disease. We show in the present study that DNAJB6 also inhibits amyloid formation by an even more aggregation-prone peptide (the amyloid-beta peptide, Aβ42, implicated in Alzheimer disease) in a highly efficient manner. By monitoring fibril formation using Thioflavin T fluorescence and far-UV CD spectroscopy, we have found that the aggregation of Aβ42 is retarded by DNAJB6 in a concentration-dependent manner, extending to very low sub-stoichiometric molar ratios of chaperone to peptide. Quantitative kinetic analysis and immunochemistry studies suggest that the high inhibitory efficiency is due to the interactions of the chaperone with aggregated forms of Aβ42 rather than the monomeric form of the peptide. This interaction prevents the growth of such species to longer fibrils and inhibits the formation of new amyloid fibrils through both primary and secondary nucleation. A low dissociation rate of DNAJB6 from Aβ42 aggregates leads to its incorporation into growing fibrils and hence to its gradual depletion from solution with time. When DNAJB6 is eventually depleted, fibril proliferation takes place, but the inhibitory activity can be prolonged by introducing DNAJB6 at regular intervals during the aggregation reaction. These results reveal the highly efficacious mode of action of this molecular chaperone against protein aggregation, and demonstrate that the role of molecular chaperones can involve interactions with multiple aggregated species leading to the inhibition of both principal nucleation pathways through which aggregates are able to form.
Keywords: Aggregation Kinetics; Alzheimer Disease; Amyloid Fibril Formation; Amyloid-beta (Aβ); Chaperone DnaJ (DnaJ); Hsp40; Inhibition Mechanism; Neurodegenerative Disease; Protein Aggregation.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Conserved S/T Residues of the Human Chaperone DNAJB6 Are Required for Effective Inhibition of Aβ42 Amyloid Fibril Formation.Biochemistry. 2018 Aug 14;57(32):4891-4902. doi: 10.1021/acs.biochem.8b00353. Epub 2018 Jul 30. Biochemistry. 2018. PMID: 30024736
-
Amyloid-β oligomers are captured by the DNAJB6 chaperone: Direct detection of interactions that can prevent primary nucleation.J Biol Chem. 2020 Jun 12;295(24):8135-8144. doi: 10.1074/jbc.RA120.013459. Epub 2020 Apr 29. J Biol Chem. 2020. PMID: 32350108 Free PMC article.
-
The C-terminal domain of the antiamyloid chaperone DNAJB6 binds to amyloid-β peptide fibrils and inhibits secondary nucleation.J Biol Chem. 2023 Nov;299(11):105317. doi: 10.1016/j.jbc.2023.105317. Epub 2023 Oct 4. J Biol Chem. 2023. PMID: 37797698 Free PMC article.
-
Emerging roles and underlying molecular mechanisms of DNAJB6 in cancer.Oncotarget. 2016 Aug 16;7(33):53984-53996. doi: 10.18632/oncotarget.9803. Oncotarget. 2016. PMID: 27276715 Free PMC article. Review.
-
The roles of HSP40/DNAJ protein family in neurodegenerative diseases.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022 Nov 25;51(5):640-646. doi: 10.3724/zdxbyxb-2021-0406. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36581576 Free PMC article. Review. English.
Cited by
-
Chaperones mainly suppress primary nucleation during formation of functional amyloid required for bacterial biofilm formation.Chem Sci. 2021 Dec 13;13(2):536-553. doi: 10.1039/d1sc05790a. eCollection 2022 Jan 5. Chem Sci. 2021. PMID: 35126986 Free PMC article.
-
An engineered monomer binding-protein for α-synuclein efficiently inhibits the proliferation of amyloid fibrils.Elife. 2019 Aug 21;8:e46112. doi: 10.7554/eLife.46112. Elife. 2019. PMID: 31389332 Free PMC article.
-
Protein Quality Control Pathways at the Crossroad of Synucleinopathies.J Parkinsons Dis. 2020;10(2):369-382. doi: 10.3233/JPD-191790. J Parkinsons Dis. 2020. PMID: 31985474 Free PMC article. Review.
-
Challenging Proteostasis: Role of the Chaperone Network to Control Aggregation-Prone Proteins in Human Disease.Adv Exp Med Biol. 2020;1243:53-68. doi: 10.1007/978-3-030-40204-4_4. Adv Exp Med Biol. 2020. PMID: 32297211 Free PMC article. Review.
-
Evaluation of the amyloid beta-GFP fusion protein as a model of amyloid beta peptides-mediated aggregation: a study of DNAJB6 chaperone.Front Mol Neurosci. 2015 Jul 27;8:40. doi: 10.3389/fnmol.2015.00040. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26283911 Free PMC article.
References
-
- Chiti F., Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 - PubMed
-
- Cohen A. S., Calkins E. (1959) Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature 183, 1202–1203 - PubMed
-
- Dobson C. M. (1999) Protein misfolding, evolution and disease. Trends Biochem. Sci 24, 329–332 - PubMed
-
- Sawaya M. R., Sambashivan S., Nelson R., Ivanova M. I., Sievers S. A., Apostol M. I., Thompson M. J., Balbirnie M., Wiltzius J. J., McFarlane H. T., Madsen A., Riekel C., Eisenberg D. (2007) Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447, 453–457 - PubMed
-
- Chiti F., Stefani M., Taddei N., Ramponi G., Dobson C. M. (2003) Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424, 805–808 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

