Neurogenin 2 mediates amyloid-β precursor protein-stimulated neurogenesis
- PMID: 25217641
- PMCID: PMC4223326
- DOI: 10.1074/jbc.M114.581918
Neurogenin 2 mediates amyloid-β precursor protein-stimulated neurogenesis
Abstract
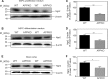
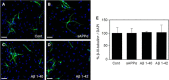
Amyloid-β precursor protein (APP) is well studied for its role in Alzheimer disease, although its normal function remains uncertain. It has been reported that APP stimulates the proliferation and neuronal differentiation of neural stem/progenitor cells (NSPCs). In this study we examined the role of APP in NSPC differentiation. To identify proteins that may mediate the effect of APP on NSPC differentiation, we used a gene array approach to find genes whose expression correlated with APP-induced neurogenesis. We found that the expression of neurogenin 2 (Ngn2), a basic helix-loop-helix transcription factor, was significantly down-regulated in NSPCs from APP knock-out mice (APPKO) and increased in APP transgenic (Tg2576) mice. Ngn2 overexpression in APPKO NSPCs promoted neuronal differentiation, whereas siRNA knockdown of Ngn2 expression in wild-type NSPCs decreased neuronal differentiation. The results demonstrate that APP-stimulated neuronal differentiation of NSPCs is mediated by Ngn2.
Keywords: Amyloid Precursor Protein (APP); Basic Helix-loop-helix Transcription Factor (bHLH); Neurogenesis; RNA Interference (RNAi); Transgenic Mice.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Laßek M., Weingarten J., Einsfelder U., Brendel P., Müller U., Volknandt W. (2013) Amyloid precursor proteins are constituents of the presynaptic active zone. J. Neurochem. 127, 48–56 - PubMed
-
- Small D. H., Clarris H. L., Williamson T. G., Reed G., Key B., Mok S. S., Beyreuther K., Masters C. L., Nurcombe V. (1999) Neurite-outgrowth regulating functions of the amyloid protein precursor of Alzheimer's disease. J. Alzheimers Dis. 1, 275–285 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

