A C-terminal membrane anchor affects the interactions of prion proteins with lipid membranes
- PMID: 25217642
- PMCID: PMC4208020
- DOI: 10.1074/jbc.M114.587345
A C-terminal membrane anchor affects the interactions of prion proteins with lipid membranes
Abstract
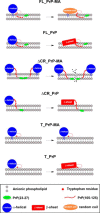
Membrane attachment via a C-terminal glycosylphosphatidylinositol anchor is critical for conversion of PrP(C) into pathogenic PrP(Sc). Therefore the effects of the anchor on PrP structure and function need to be deciphered. Three PrP variants, including full-length PrP (residues 23-231, FL_PrP), N-terminally truncated PrP (residues 90-231, T_PrP), and PrP missing its central hydrophobic region (Δ105-125, ΔCR_PrP), were equipped with a C-terminal membrane anchor via a semisynthesis strategy. Analyses of the interactions of lipidated PrPs with phospholipid membranes demonstrated that C-terminal membrane attachment induces a different binding mode of PrP to membranes, distinct from that of non-lipidated PrPs, and influences the biochemical and conformational properties of PrPs. Additionally, fluorescence-based assays indicated pore formation by lipidated ΔCR_PrP, a variant that is known to be highly neurotoxic in transgenic mice. This finding was supported by using patch clamp electrophysiological measurements of cultured cells. These results provide new evidence for the role of the membrane anchor in PrP-lipid interactions, highlighting the importance of the N-terminal and the central hydrophobic domain in these interactions.
Keywords: Liposome; Membrane Anchor; Pore Formation; Prion; Protein Aggregation; Protein Chemistry; Protein Conformation; Protein Semisynthesis; lipid-Protein Interactions.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Synthesis and structural characterization of a mimetic membrane-anchored prion protein.FEBS J. 2006 Mar;273(6):1285-99. doi: 10.1111/j.1742-4658.2006.05152.x. FEBS J. 2006. PMID: 16519692
-
Binding of prion protein to lipid membranes and implications for prion conversion.J Mol Biol. 2002 Feb 1;315(5):1241-56. doi: 10.1006/jmbi.2001.5322. J Mol Biol. 2002. PMID: 11827491
-
Cytoskeleton-dependent clustering of membrane-bound prion protein on the cell surface.J Biol Chem. 2021 Jan-Jun;296:100359. doi: 10.1016/j.jbc.2021.100359. Epub 2021 Feb 2. J Biol Chem. 2021. PMID: 33539927 Free PMC article.
-
Aggregation and fibrillization of prions in lipid membranes.Biochem Soc Symp. 2005;(72):211-22. doi: 10.1042/bss0720211. Biochem Soc Symp. 2005. PMID: 15649144 Review.
-
Biochemical Characterization of Prions.Prog Mol Biol Transl Sci. 2017;150:389-407. doi: 10.1016/bs.pmbts.2017.06.012. Epub 2017 Aug 8. Prog Mol Biol Transl Sci. 2017. PMID: 28838671 Review.
Cited by
-
Segmental Isotope Labeling of the Prion Protein: Identification of a Key Residue for Copper-Mediated Interdomain Structure.ACS Chem Biol. 2025 Aug 15;20(8):1980-1989. doi: 10.1021/acschembio.5c00336. Epub 2025 Jul 24. ACS Chem Biol. 2025. PMID: 40704886 Free PMC article.
-
Chemische Synthese und Semisynthese von lipidierten Proteinen.Angew Chem Weinheim Bergstr Ger. 2022 Apr 4;134(15):e202111266. doi: 10.1002/ange.202111266. Epub 2022 Feb 3. Angew Chem Weinheim Bergstr Ger. 2022. PMID: 38504765 Free PMC article. Review.
-
Akt Kinase Activation Mechanisms Revealed Using Protein Semisynthesis.Cell. 2018 Aug 9;174(4):897-907.e14. doi: 10.1016/j.cell.2018.07.003. Epub 2018 Aug 2. Cell. 2018. PMID: 30078705 Free PMC article.
-
Semisynthetic prion protein (PrP) variants carrying glycan mimics at position 181 and 197 do not form fibrils.Chem Sci. 2017 Sep 1;8(9):6626-6632. doi: 10.1039/c7sc02719b. Epub 2017 Jul 24. Chem Sci. 2017. PMID: 28989689 Free PMC article.
-
The prion protein family member Shadoo induces spontaneous ionic currents in cultured cells.Sci Rep. 2016 Nov 7;6:36441. doi: 10.1038/srep36441. Sci Rep. 2016. PMID: 27819308 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

