Structural determinants and proliferative consequences of connexin 37 hemichannel function in insulinoma cells
- PMID: 25217644
- PMCID: PMC4215222
- DOI: 10.1074/jbc.M114.583054
Structural determinants and proliferative consequences of connexin 37 hemichannel function in insulinoma cells
Abstract
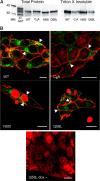
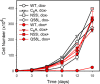
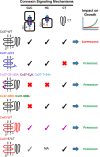
Connexin (Cx) 37 suppresses vascular and cancer cell proliferation. The C terminus and a channel able to function are necessary, and neither by itself is sufficient, for Cx37 to mediate growth suppression. Cx37 supports transmembrane and intercellular signaling by forming functional hemichannels (HCs) and gap junction channels (GJCs), respectively. Here we determined whether Cx37 with HC, but not GJC, functionality would suppress proliferation of rat insulinoma (Rin) cells comparably to wild-type Cx37 (Cx37-WT). We mutated extracellular loop residues hypothesized to compromise HC docking but not HC function (six cysteines mutated to alanine, C54A,C61A,C65A, C187A,C192A,C198A (designated as C6A); N55I; and Q58L). All three mutants trafficked to the plasma membrane and formed protein plaques comparably to Cx37-WT. None of the mutants formed functional GJCs, and Cx37-C6A did not form functional HCs. Cx37-N55I and -Q58L formed HCs with behavior and permeation properties similar to Cx37-WT (especially Q58L), but none of the mutants suppressed Rin cell proliferation. The data indicate that determinants of Cx37 HC function differ from other Cxs and that HC functions with associated HC-supported protein-protein interactions are not sufficient for Cx37 to suppress Rin cell proliferation. Together with previously published data, these results suggest that Cx37 suppresses Rin cell proliferation only when in a specific conformation achieved by interaction of the C terminus with a Cx37 pore-forming domain able to open as a GJC.
Keywords: Arterial Endothelium; Cell Cycle Control; Cell-Cell Interaction; Connexin; Gap Junction; Hemichannel; Intercellular Communication; Proliferation; Protein Targeting.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Extracellular loop cysteine mutant of cx37 fails to suppress proliferation of rat insulinoma cells.J Membr Biol. 2012 Jul;245(7):369-80. doi: 10.1007/s00232-012-9459-x. Epub 2012 Jul 15. J Membr Biol. 2012. PMID: 22797939 Free PMC article.
-
Carboxy terminus and pore-forming domain properties specific to Cx37 are necessary for Cx37-mediated suppression of insulinoma cell proliferation.Am J Physiol Cell Physiol. 2013 Dec 15;305(12):C1246-56. doi: 10.1152/ajpcell.00159.2013. Epub 2013 Oct 16. Am J Physiol Cell Physiol. 2013. PMID: 24133065 Free PMC article.
-
A functional channel is necessary for growth suppression by Cx37.J Cell Sci. 2011 Jul 15;124(Pt 14):2448-56. doi: 10.1242/jcs.081695. Epub 2011 Jun 21. J Cell Sci. 2011. PMID: 21693589 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease.Neuropharmacology. 2013 Dec;75:479-90. doi: 10.1016/j.neuropharm.2013.03.040. Epub 2013 Apr 12. Neuropharmacology. 2013. PMID: 23587648 Review.
Cited by
-
New Insights into Pulmonary Hypertension: A Role for Connexin-Mediated Signalling.Int J Mol Sci. 2021 Dec 29;23(1):379. doi: 10.3390/ijms23010379. Int J Mol Sci. 2021. PMID: 35008804 Free PMC article. Review.
-
Serine 319 phosphorylation is necessary and sufficient to induce a Cx37 conformation that leads to arrested cell cycling.J Cell Sci. 2020 Jun 18;133(12):jcs240721. doi: 10.1242/jcs.240721. J Cell Sci. 2020. PMID: 32350069 Free PMC article.
-
Extracellular Cysteine in Connexins: Role as Redox Sensors.Front Physiol. 2016 Jan 28;7:1. doi: 10.3389/fphys.2016.00001. eCollection 2016. Front Physiol. 2016. PMID: 26858649 Free PMC article.
-
Connexin and pannexin channels in cancer.BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):12. doi: 10.1186/s12860-016-0094-8. BMC Cell Biol. 2016. PMID: 27229305 Free PMC article. Review.
-
Domain-specific distribution of gap junctions defines cellular coupling to establish a vascular relay in the retina.J Comp Neurol. 2019 Nov 1;527(16):2675-2693. doi: 10.1002/cne.24699. Epub 2019 Apr 13. J Comp Neurol. 2019. PMID: 30950036 Free PMC article.
References
-
- Söhl G., Willecke K. (2004) Gap junctions and the connexin protein family. Cardiovasc. Res. 62, 228–232 - PubMed
-
- Goodenough D. A., Paul D. L. (2003) Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 4, 285–294 - PubMed
-
- Harris A. L. (2001) Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 34, 325–472 - PubMed
-
- Kardami E., Dang X., Iacobas D. A., Nickel B. E., Jeyaraman M., Srisakuldee W., Makazan J., Tanguy S., Spray D. C. (2007) The role of connexins in controlling cell growth and gene expression. Prog. Biophys. Mol. Biol. 94, 245–264 - PubMed
-
- Vinken M., Decrock E., Leybaert L., Bultynck G., Himpens B., Vanhaecke T., Rogiers V. (2012) Non-channel functions of connexins in cell growth and cell death. Biochim. Biophys. Acta 1818, 2002–2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

