GPR126 protein regulates developmental and pathological angiogenesis through modulation of VEGFR2 receptor signaling
- PMID: 25217645
- PMCID: PMC4263886
- DOI: 10.1074/jbc.M114.571000
GPR126 protein regulates developmental and pathological angiogenesis through modulation of VEGFR2 receptor signaling
Abstract
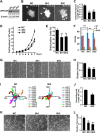
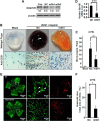
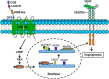
Angiogenesis, the formation of new blood vessels from pre-existing ones, is essential for development, wound healing, and tumor progression. The VEGF pathway plays irreplaceable roles during angiogenesis, but how other signals cross-talk with and modulate VEGF cascades is not clearly elucidated. Here, we identified that Gpr126, an endothelial cell-enriched gene, plays an important role in angiogenesis by regulating endothelial cell proliferation, migration, and tube formation. Knockdown of Gpr126 in the mouse retina resulted in the inhibition of hypoxia-induced angiogenesis. Interference of Gpr126 expression in zebrafish embryos led to defects in intersegmental vessel formation. Finally, we identified that GPR126 regulated the expression of VEGFR2 by targeting STAT5 and GATA2 through the cAMP-PKA-cAMP-response element-binding protein signaling pathway during angiogenesis. Our findings illustrate that GPR126 modulates both physiological and pathological angiogenesis through VEGF signaling, providing a potential target for the treatment of angiogenesis-related diseases.
Keywords: Angiogenesis; Endothelial Cell; G Protein-coupled Receptor (GPCR); Protein Kinase A (PKA); VEGFR2; Vascular Endothelial Growth Factor (VEGF).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis.J Biol Chem. 2013 Jul 19;288(29):21184-21196. doi: 10.1074/jbc.M113.481119. Epub 2013 Jun 2. J Biol Chem. 2013. PMID: 23729680 Free PMC article.
-
Integrin alpha x stimulates cancer angiogenesis through PI3K/Akt signaling-mediated VEGFR2/VEGF-A overexpression in blood vessel endothelial cells.J Cell Biochem. 2019 Feb;120(2):1807-1818. doi: 10.1002/jcb.27480. Epub 2018 Sep 14. J Cell Biochem. 2019. PMID: 30873824
-
SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo.Clin Cancer Res. 2011 Jul 1;17(13):4439-50. doi: 10.1158/1078-0432.CCR-10-3109. Epub 2011 May 27. Clin Cancer Res. 2011. PMID: 21622720
-
Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis.J Biochem Mol Biol. 2006 Sep 30;39(5):469-78. doi: 10.5483/bmbrep.2006.39.5.469. J Biochem Mol Biol. 2006. PMID: 17002866 Review.
-
The regulatory role of microRNAs in angiogenesis-related diseases.J Cell Mol Med. 2018 Oct;22(10):4568-4587. doi: 10.1111/jcmm.13700. Epub 2018 Jun 29. J Cell Mol Med. 2018. PMID: 29956461 Free PMC article. Review.
Cited by
-
Gpr126/Adgrg6 contributes to the terminal Schwann cell response at the neuromuscular junction following peripheral nerve injury.Glia. 2020 Jun;68(6):1182-1200. doi: 10.1002/glia.23769. Epub 2019 Dec 24. Glia. 2020. PMID: 31873966 Free PMC article.
-
Phenotypic expansion illuminates multilocus pathogenic variation.Genet Med. 2018 Dec;20(12):1528-1537. doi: 10.1038/gim.2018.33. Epub 2018 Apr 26. Genet Med. 2018. PMID: 29790871 Free PMC article.
-
Cartilage organoids for cartilage development and cartilage-associated disease modeling.Front Cell Dev Biol. 2023 Jan 30;11:1125405. doi: 10.3389/fcell.2023.1125405. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36824369 Free PMC article.
-
Recent progress in human placental transcriptomics.Dev Period Med. 2019;23(2):104-108. doi: 10.34763/devperiodmed.20192302.104108. Dev Period Med. 2019. PMID: 31280246 Free PMC article.
-
Aggressive Head and Neck Squamous Cell Carcinoma in the Setting of GATA2 Deficiency.Head Neck. 2025 Jul;47(7):E82-E86. doi: 10.1002/hed.28117. Epub 2025 Feb 21. Head Neck. 2025. PMID: 39981594 Free PMC article.
References
-
- Schmidt A., Brixius K., Bloch W. (2007) Endothelial precursor cell migration during vasculogenesis. Circ. Res. 101, 125–136 - PubMed
-
- Pardanaud L., Luton D., Prigent M., Bourcheix L. M., Catala M., Dieterlen-Lievre F. (1996) Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development 122, 1363–1371 - PubMed
-
- Pober J. S., Sessa W. C. (2007) Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 - PubMed
-
- Yancopoulos G. D., Davis S., Gale N. W., Rudge J. S., Wiegand S. J., Holash J. (2000) Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

