Chronic exposure to perfluorinated compounds: Impact on airway hyperresponsiveness and inflammation
- PMID: 25217661
- PMCID: PMC4233295
- DOI: 10.1152/ajplung.00100.2014
Chronic exposure to perfluorinated compounds: Impact on airway hyperresponsiveness and inflammation
Abstract
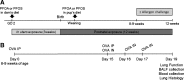
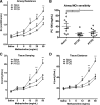
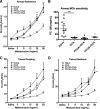
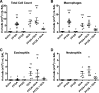
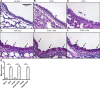
Emerging epidemiological evidence reveals a link between lung disease and exposure to indoor pollutants such as perfluorinated compounds (PFCs). PFC exposure during critical developmental stages may increase asthma susceptibility. Thus, in a murine model, we tested the hypothesis that early life and continued exposure to two ubiquitous household PFCs, perfluorooctanoic acid (PFOA) and perflurooctanesulfonic acid (PFOS), can induce lung dysfunction that exacerbates allergen-induced airway hyperresponsiveness (AHR) and inflammation. Balb/c mice were exposed to PFOA or PFOS (4 mg/kg chow) from gestation day 2 to 12 wk of age by feeding pregnant and nursing dams, and weaned pups. Some pups were also sensitized and challenged with ovalbumin (OVA). We assessed lung function and inflammatory cell and cytokine expression in the lung and examined bronchial goblet cell number. PFOA, but not PFOS, without the OVA sensitization/challenge induced AHR concomitant with a 25-fold increase of lung macrophages. PFOA exposure did not affect OVA-induced lung inflammatory cell number. In contrast, PFOS exposure inhibited OVA-induced lung inflammation, decreasing total cell number in lung lavage by 68.7%. Interferon-γ mRNA in the lung was elevated in all PFC-exposed groups. Despite these effects, neither PFOA nor PFOS affected OVA-induced AHR. Our data do not reveal PFOA or PFOS exposure as a risk factor for more severe allergic asthma-like symptoms, but PFOA alone can induce airway inflammation and alter airway function.
Keywords: asthma; environmental pollutant; interferon-γ; perfluorooctanoic acid; perflurooctanesulfonic acid.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Anderson-Mahoney P, Kotlerman J, Takhar H, Gray D, Dahlgren J. Self-reported health effects among community residents exposed to perfluorooctanoate. J Environ Occup Health Policy 18: 129–143, 2008. - PubMed
-
- Arai N, Kondo M, Izumo T, Tamaoki J, Nagai A. Inhibition of neutrophil elastase-induced goblet cell metaplasia by tiotropium in mice. Eur Resp J 35: 1164–1171, 2010. - PubMed
-
- Chan E, Burstyn I, Cherry N, Bamforth F, Martin JW. Perfluorinated acids and hypothyroxinemia in pregnant women. Environ Res 111: 559–564, 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

