Discussions about clinical trials among patients with newly diagnosed lung and colorectal cancer
- PMID: 25217775
- PMCID: PMC4168309
- DOI: 10.1093/jnci/dju216
Discussions about clinical trials among patients with newly diagnosed lung and colorectal cancer
Abstract
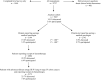
Background: Clinical trials are essential to establish the effectiveness of new cancer therapies, but less than 5% of adults with cancer enroll in trials. In addition to ineligibility or lack of available trials, barriers to enrollment may include limited patient awareness about the option of participation.
Methods: We surveyed a multiregional cohort of patients with lung or colorectal cancer (or their surrogates) three to six months after diagnosis. We assessed whether respondents reported learning that clinical trial participation might be an option, and, if so, with whom they discussed trials. We used logistic regression to assess the association of patient characteristics with discussing trial participation and enrolling in trials. All statistical tests were two-sided.
Results: Of 7887 respondents, 1114 (14.1%) reported discussing the possibility of clinical trial participation; most learned about trials from their physicians, and 287 patients (3.6% of all patients, 25.8% of trial discussants) enrolled. Among 2173 patients who received chemotherapy for advanced (stage III/IV lung or stage IV colorectal) cancer, 25.7% discussed trials, and 7.6% (29.5% of trial discussants) enrolled. Discussions were less frequent among older patients, African American or Asian vs white patients, and those with lower incomes and more comorbidity. Enrollment was higher among patients reporting shared vs physician-driven decisions (all P < .05).
Conclusions: In this population-based cohort, only 14% of patients discussed participation in clinical trials. Discussions were more frequent among advanced cancer patients but were still reported by a minority of patients. Strategies to expand access to trials and facilitate patient-provider communication about participation may accelerate development of better cancer therapeutics.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Discussions about clinical trials among patients with newly diagnosed lung and colorectal cancer.J Natl Cancer Inst. 2014 Sep 13;106(10):dju265. doi: 10.1093/jnci/dju265. Print 2014 Oct. J Natl Cancer Inst. 2014. PMID: 25217777 No abstract available.
Similar articles
-
Tumor board participation among physicians caring for patients with lung or colorectal cancer.J Oncol Pract. 2015 May;11(3):e267-78. doi: 10.1200/JOP.2015.003673. Epub 2015 Apr 28. J Oncol Pract. 2015. PMID: 25922221 Free PMC article.
-
Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care.JAMA Oncol. 2015 Apr;1(1):50-8. doi: 10.1001/jamaoncol.2014.112. JAMA Oncol. 2015. PMID: 26182303 Free PMC article.
-
Discordant attitudes and beliefs about cancer clinical trial participation between physicians, research staff, and cancer patients.Clin Trials. 2020 Apr;17(2):184-194. doi: 10.1177/1740774520901514. Epub 2020 Feb 3. Clin Trials. 2020. PMID: 32009456 Free PMC article.
-
Involving South Asian patients in clinical trials.Health Technol Assess. 2004 Oct;8(42):iii, 1-109. doi: 10.3310/hta8420. Health Technol Assess. 2004. PMID: 15488164 Review.
-
Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation.J Natl Cancer Inst. 2019 Mar 1;111(3):245-255. doi: 10.1093/jnci/djy221. J Natl Cancer Inst. 2019. PMID: 30856272 Free PMC article.
Cited by
-
Randomised controlled trials as part of clinical care: A seven-step routinisation framework proposal.Int Wound J. 2019 Apr;16(2):442-458. doi: 10.1111/iwj.13053. Epub 2018 Dec 19. Int Wound J. 2019. PMID: 30565877 Free PMC article.
-
Kidney cancer survivorship care: Patient experiences in a Canadian setting.Can Urol Assoc J. 2020 Nov;14(11):E560-E567. doi: 10.5489/cuaj.6217. Can Urol Assoc J. 2020. PMID: 32520710 Free PMC article.
-
Insurance Clearance for Early-Phase Oncology Clinical Trials Following the Affordable Care Act.Clin Cancer Res. 2017 Aug 1;23(15):4155-4162. doi: 10.1158/1078-0432.CCR-16-3027. Epub 2017 Jul 20. Clin Cancer Res. 2017. PMID: 28729355 Free PMC article.
-
Recruitment practices for U.S. minority and underserved populations in NRG oncology: Results of an online survey.Contemp Clin Trials Commun. 2018 Mar 9;10:100-104. doi: 10.1016/j.conctc.2018.03.003. eCollection 2018 Jun. Contemp Clin Trials Commun. 2018. PMID: 30023443 Free PMC article.
-
Nano-Theranostics for the Sensing, Imaging and Therapy of Prostate Cancers.Front Chem. 2022 Apr 12;10:830133. doi: 10.3389/fchem.2022.830133. eCollection 2022. Front Chem. 2022. PMID: 35494646 Free PMC article. Review.
References
-
- Nass SJ, Moses HL, Mendelsohn J. A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative Group Program. Committee on Cancer Clinical Trials and the NCI Cooperative Group Program; Institute of Medicine, National Academies Press; 2010:1371. - PubMed
-
- Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–3334 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical