Inhibition of 32Dp210 cells harboring T315I mutation by a novel derivative of emodin correlates with down-regulation of BCR-ABL and its downstream signaling pathways
- PMID: 25217883
- PMCID: PMC11823672
- DOI: 10.1007/s00432-014-1820-2
Inhibition of 32Dp210 cells harboring T315I mutation by a novel derivative of emodin correlates with down-regulation of BCR-ABL and its downstream signaling pathways
Abstract
Purpose: The clinical outcome of chronic myeloid leukemia (CML) patients has been changed dramatically due to the development of imatinib (IM). However, the emergence of IM resistance, commonly associated with point mutations within the BCR-ABL kinase domain, remains a major clinical problem. Here, we investigated the effects of E35, a novel derivative of emodin, on the IM-resistant 32Dp210-T315I cells.
Methods: Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide and colony formation assay. Induction of apoptosis was confirmed by DNA fragmentation assay and annexin V/PI staining assay. Real-time quantitative PCR was used to access the BCR-ABL gene expression. Changes of related signaling molecules were detected through Western blot.
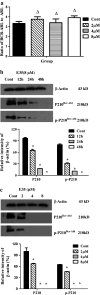
Results: E35 was found to potently inhibit proliferation of 32Dp210-T315I cells with an average IC50 of 2.4 µM at 48 h. Colony formation was almost fully suppressed in 1.0 μM E35 group. DNA fragmentation and annexin V/PI staining assay exhibited the typical DNA fragmentation and the increased proportion of early apoptotic cells, respectively. The induction of apoptosis was associated with increase of Bax to Bcl-2 expression ratio and activation of caspase cascades involving decrease of pro-caspase 9 and pro-caspase 3 and increase of PARP cleavage. The protein expression of P210(BCR-ABL) and p-P210(BCR-ABL) was down-regulated in the presence of E35, although the mRNA levels remained almost unchanged. Moreover, the activation of the P210(BCR-ABL) downstream signaling pathways including CrkL, Akt/mTOR and MEK/ERK was fully suppressed by E35.
Conclusion: Our study indicated that E35 might be a potential antileukemia agent against IM resistance in CML.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




Similar articles
-
Enhanced ABL-inhibitor-induced MAPK-activation in T315I-BCR-ABL-expressing cells: a potential mechanism of altered leukemogenicity.J Cancer Res Clin Oncol. 2012 Feb;138(2):203-12. doi: 10.1007/s00432-011-1086-x. Epub 2011 Nov 17. J Cancer Res Clin Oncol. 2012. PMID: 22089930 Free PMC article.
-
Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-κB modulation.J Cancer Res Clin Oncol. 2012 Dec;138(12):2095-102. doi: 10.1007/s00432-012-1292-1. Epub 2012 Jul 26. J Cancer Res Clin Oncol. 2012. PMID: 22833150 Free PMC article.
-
Curcumin derivative C817 inhibits proliferation of imatinib-resistant chronic myeloid leukemia cells with wild-type or mutant Bcr-Abl in vitro.Acta Pharmacol Sin. 2014 Mar;35(3):401-9. doi: 10.1038/aps.2013.180. Epub 2014 Feb 3. Acta Pharmacol Sin. 2014. PMID: 24487968 Free PMC article.
-
Chronic myelogenous leukemia: molecular and cellular aspects.J Cancer Res Clin Oncol. 1998;124(12):643-60. doi: 10.1007/s004320050228. J Cancer Res Clin Oncol. 1998. PMID: 9879825 Free PMC article. Review.
-
Dasatinib and nilotinib for imatinib-resistant or -intolerant chronic myeloid leukaemia: a systematic review and economic evaluation.Health Technol Assess. 2012;16(22):1-410. doi: 10.3310/hta16220. Health Technol Assess. 2012. PMID: 22551803 Free PMC article.
Cited by
-
In Vitro Investigation of the Cytotoxic Activity of Emodin 35 Derivative on Multiple Myeloma Cell Lines.Evid Based Complement Alternat Med. 2021 Jan 25;2021:6682787. doi: 10.1155/2021/6682787. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33564319 Free PMC article.
-
Integrated bioinformatics analysis of the crucial candidate genes and pathways associated with glucocorticoid resistance in acute lymphoblastic leukemia.Cancer Med. 2020 Apr;9(8):2918-2929. doi: 10.1002/cam4.2934. Epub 2020 Feb 25. Cancer Med. 2020. PMID: 32096603 Free PMC article.
-
Doxorubicin/Nucleophosmin Binding Protein-Conjugated Nanoparticle Enhances Anti-leukemia Activity in Acute Lymphoblastic Leukemia Cells in vitro and in vivo.Front Pharmacol. 2021 May 28;12:607755. doi: 10.3389/fphar.2021.607755. eCollection 2021. Front Pharmacol. 2021. PMID: 34122059 Free PMC article.
-
Antitumor Effects and Mechanism of Novel Emodin Rhamnoside Derivatives against Human Cancer Cells In Vitro.PLoS One. 2015 Dec 18;10(12):e0144781. doi: 10.1371/journal.pone.0144781. eCollection 2015. PLoS One. 2015. PMID: 26682731 Free PMC article.
-
E35 ablates acute leukemia stem and progenitor cells in vitro and in vivo.J Cell Physiol. 2020 Nov;235(11):8023-8034. doi: 10.1002/jcp.29457. Epub 2020 Jan 21. J Cell Physiol. 2020. PMID: 31960417 Free PMC article.
References
-
- Apperley JF (2007) Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol 8(11):1018–1029. doi:10.1016/S1470-2045(07)70342-X - PubMed
-
- Branford S, Hughes TP (2011) Mutational analysis in chronic myeloid leukemia: when and what to do. Curr Opin Hematol 18(2):111–116. doi:10.1097/MOH.0b013e32834399ef - PubMed
-
- Branford S, Melo JV, Hughes TP (2009) Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter. Blood 114(27):5426–5435. doi:10.1182/blood-2009-08-215939 - PubMed
-
- Chen YY, Li J, Hu JD, Zheng J, Zheng ZH, Zhu LF, Chen XJ, Lin ZX (2013) Reversing effects of emodin on multidrug resistance in resistant HL-60/ADR cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 21(6):1413–1422 - PubMed
-
- Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, O’Hare T, Hu S, Narasimhan NI, Rivera VM, Clackson T, Turner CD, Haluska FG, Druker BJ, Deininger MW, Talpaz M (2012) Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med 367(22):2075–2088. doi:10.1056/NEJMoa1205127 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

