Energetic dissection of Gleevec's selectivity toward human tyrosine kinases
- PMID: 25218445
- PMCID: PMC4266587
- DOI: 10.1038/nsmb.2891
Energetic dissection of Gleevec's selectivity toward human tyrosine kinases
Abstract
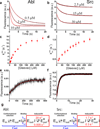
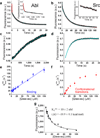
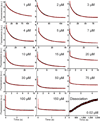
Protein kinases are obvious drug targets against cancer, owing to their central role in cellular regulation. Since the discovery of Gleevec, a potent and specific inhibitor of Abl kinase, as a highly successful cancer therapeutic, the ability of this drug to distinguish between Abl and other tyrosine kinases such as Src has been intensely investigated but without much success. Using NMR and fast kinetics, we establish a new model that solves this longstanding question of how the two tyrosine kinases adopt almost identical structures when bound to Gleevec but have vastly different affinities. We show that, in contrast to all other proposed models, the origin of Abl's high affinity lies predominantly in a conformational change after binding. An energy landscape providing tight affinity via an induced fit and binding plasticity via a conformational-selection mechanism is likely to be general for many inhibitors.
Figures


References
-
- Cohen P. Protein kinases--the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. - PubMed
ONLINE METHODS REFERENCES
-
- Ericsson UB, Hallberg BM, Detitta GT, Dekker N, Nordlund P. Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal Biochem. 2006;357:289–298. - PubMed
-
- Delaglio F, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. - PubMed
-
- Vranken WF, et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005;59:687–696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

