Cost-effectiveness of sofosbuvir in the treatment of patients with hepatitis C
- PMID: 25219291
- PMCID: PMC4406130
- DOI: 10.1111/jvh.12311
Cost-effectiveness of sofosbuvir in the treatment of patients with hepatitis C
Abstract
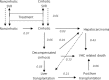
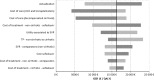
In France, 190,306 patients were suffering from chronic hepatitis C in 2012. These patients have a decreased life expectancy and are susceptible to complications associated with chronic hepatitis. Current treatments are poorly tolerated and their effectiveness varies depending on the genotype of the virus. Sofosbuvir, a new class of treatment, has demonstrated in five phase III trials sustained viral response (SVR) rates of over 90% across genotypes, higher than current treatments and has a tolerance profile similar to placebo. The objective was to determine the cost-effectiveness of using sofosbuvir in the treatment of chronic HCV infection. A Markov model was used to compare treatment strategies with and without sofosbuvir. The model simulated the natural history of HCV infection. SVR rates were based on data from clinical trials. Utilities associated with different stages of disease were based on data from the literature. French direct medical costs were used. Price for sofosbuvir was the price used in the early access program for severe fibrosis stages. The incremental cost-effectiveness ratio for sofosbuvir versus current reference treatments was € 16,278/QALY and varied from 40,000 €/QALY for F0 stages to 12,080 €/QALY for F4 stages. The sensitivity analyses carried out confirmed the robustness of this result. Sofosbuvir is a cost-effective treatment option for patients with hepatitis C.
Keywords: France; cost-benefit analysis; healthcare costs; hepatitis C; sofosbuvir.
© 2014 The Authors. Journal of Viral Hepatitis Published by John Wiley & Sons Ltd.
Figures
References
-
- Alter MJ. Epidemiology of hepatitis C. Hepatol Baltim Md. 1997;26(3 Suppl 1):62S–65S. - PubMed
-
- INVS. 2009. [National reference lab data for hepatitis C, 2001-2007] Surveillance nationale de l'hépatite C à partir des pôles de référence, données épidémiologiques 2001-2007. Saint-Maurice Cedex, France Institut de Veille Sanitaire.
-
- Meffre C, Le Strat Y, Delarocque-Astagneau E, et al. Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: social factors are important predictors after adjusting for known risk factors. J Med Virol. 2010;82(4):546–555. - PubMed
-
- Deuffic-Burban S, Mathurin P, Pol S, et al. Impact of hepatitis C triple therapy availability upon the number of patients to be treated and associated costs in France: a model-based analysis. Gut. 2012;61(2):290–296. - PubMed
-
- Henri L, Homie R, Chris E, Christophe H, Martin B, Francoise R-T. Modeling the epidemiological impact of upcoming direct antiviral agents in hepatitis C treatment. Hepatology. 2013;58:208A–1309A.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources