New considerations in radiation treatment planning for brain tumors: neural progenitor cell-containing niches
- PMID: 25219811
- PMCID: PMC4467686
- DOI: 10.1016/j.semradonc.2014.06.007
New considerations in radiation treatment planning for brain tumors: neural progenitor cell-containing niches
Erratum in
- Semin Radiat Oncol. 2015 Apr;25(2):151
Abstract
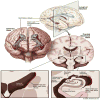
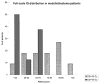
The purpose of this critical review is to explore the controversy regarding the relationship between radiation dose to the neural progenitor cell (NPC) niches and patient outcomes, in terms of both toxicity and tumor control. NPCs in the subventricular zone (SVZ) and hippocampus are paradoxically associated with long-term neurocognitive sequelae of brain irradiation, as well as resistance to therapy and tumor recurrence. The reconciliation of these somewhat opposing functions is challenging. Current literature suggests that radiation and other treatments against the NPC in the hippocampus and the SVZ may influence patient outcome. As a result, both the SVZ and the hippocampus could have important implications on radiation treatment planning strategies, and future laboratory and clinical evaluations will be critical in designing studies to optimize treatment outcome, effectiveness, and safety.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
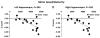


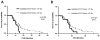
Similar articles
-
A radiotherapy technique to limit dose to neural progenitor cell niches without compromising tumor coverage.J Neurooncol. 2011 Sep;104(2):579-87. doi: 10.1007/s11060-011-0530-8. Epub 2011 Feb 14. J Neurooncol. 2011. PMID: 21327710 Free PMC article.
-
A Prospective Cohort Study of Neural Progenitor Cell-Sparing Radiation Therapy Plus Temozolomide for Newly Diagnosed Patients With Glioblastoma.Neurosurgery. 2020 Jul 1;87(1):E31-E40. doi: 10.1093/neuros/nyaa107. Neurosurgery. 2020. PMID: 32497183 Free PMC article.
-
Neural stem cell sparing by linac based intensity modulated stereotactic radiotherapy in intracranial tumors.Radiat Oncol. 2013 Jul 24;8:187. doi: 10.1186/1748-717X-8-187. Radiat Oncol. 2013. PMID: 23883368 Free PMC article.
-
[Radiotherapy of brain tumors. New techniques and treatment strategies for].Nervenarzt. 2010 Aug;81(8):918, 920-4, 926-7. doi: 10.1007/s00115-010-2955-2. Nervenarzt. 2010. PMID: 20669005 Review. German.
-
Stem cell niche irradiation in glioblastoma: providing a ray of hope?CNS Oncol. 2014;3(5):367-76. doi: 10.2217/cns.14.39. CNS Oncol. 2014. PMID: 25363009 Free PMC article. Review.
Cited by
-
Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma.Strahlenther Onkol. 2016 Nov;192(11):759-769. doi: 10.1007/s00066-016-1005-9. Epub 2016 Jun 30. Strahlenther Onkol. 2016. PMID: 27363701 Free PMC article.
-
Neural stem cells, the subventricular zone and radiotherapy: implications for treating glioblastoma.J Neurooncol. 2016 Jun;128(2):207-16. doi: 10.1007/s11060-016-2123-z. Epub 2016 Apr 23. J Neurooncol. 2016. PMID: 27108274 Review.
-
Comparison of the Effectiveness of Radiotherapy with 3D-CRT, IMRT, VMAT and PT for Newly Diagnosed Glioblastoma: A Bayesian Network Meta-Analysis.Cancers (Basel). 2023 Dec 3;15(23):5698. doi: 10.3390/cancers15235698. Cancers (Basel). 2023. PMID: 38067401 Free PMC article.
-
Repeatability of radiotherapy dose-painting prescriptions derived from a multiparametric magnetic resonance imaging model of glioblastoma infiltration.Phys Imaging Radiat Oncol. 2022 Jun 11;23:8-15. doi: 10.1016/j.phro.2022.06.004. eCollection 2022 Jul. Phys Imaging Radiat Oncol. 2022. PMID: 35734265 Free PMC article.
-
Radiotherapy-Induced Neurocognitive Dysfunction in Brain Tumor Survivors: Burden and Rehabilitation.Acta Neurochir Suppl. 2023;130:197-206. doi: 10.1007/978-3-030-12887-6_24. Acta Neurochir Suppl. 2023. PMID: 37548740
References
-
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. - PubMed
-
- DeAngelis LM, Delattre J-Y, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789. - PubMed
-
- Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Physi. 2008;72:1311–1318. - PubMed
-
- Fisher B, Seiferheld W, Schultz C, et al. Secondary analysis of Radiation Therapy Oncology Group study (RTOG) 9310: An intergroup phase II combined modality treatment of primary central nervous system lymphoma. J Neurooncol. 2005;74:201–205. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

