Planar bioadhesive microdevices: a new technology for oral drug delivery
- PMID: 25219863
- PMCID: PMC4227534
- DOI: 10.2174/1389201015666140915152706
Planar bioadhesive microdevices: a new technology for oral drug delivery
Abstract
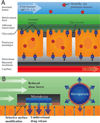
The oral route is the most convenient and least expensive route of drug administration. Yet, it is accompanied by many physiological barriers to drug uptake including low stomach pH, intestinal enzymes and transporters, mucosal barriers, and high intestinal fluid shear. While many drug delivery systems have been developed for oral drug administration, the physiological components of the gastro intestinal tract remain formidable barriers to drug uptake. Recently, microfabrication techniques have been applied to create micron-scale devices for oral drug delivery with a high degree of control over microdevice size, shape, chemical composition, drug release profile, and targeting ability. With precise control over device properties, microdevices can be fabricated with characteristics that provide increased adhesion for prolonged drug exposure, unidirectional release which serves to avoid luminal drug loss and enhance drug permeation, and protection of a drug payload from the harsh environment of the intestinal tract. Here we review the recent developments in microdevice technology and discuss the potential of these devices to overcome unsolved challenges in oral drug delivery.
Conflict of interest statement
None declared.
Figures





References
-
- International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nature reviews. Drug discovery. 2010;9(3):215–236. - PMC - PubMed
-
- Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: challenges and opportunities. Journal of controlled release : official journal of the Controlled Release Society. 2013;170(1):15–40. - PubMed
-
- Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nature reviews. Drug discovery. 2003;2(4):289–295. - PubMed
-
- Gupta S, Jain A, Chakraborty M, Sahni JK, Ali J, Dang S. Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug delivery. 2013;20(6):237–246. - PubMed
-
- Agrawal U, Sharma R, Gupta M, Vyas SP. Is nanotechnology a boon for oral drug delivery? Drug discovery today. 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

