A longitudinal study evaluating the effects of interferon-alpha therapy on cognitive and psychiatric function in adults with chronic hepatitis C
- PMID: 25219976
- PMCID: PMC4435678
- DOI: 10.1016/j.jpsychores.2014.07.020
A longitudinal study evaluating the effects of interferon-alpha therapy on cognitive and psychiatric function in adults with chronic hepatitis C
Abstract
Objective: To prospectively evaluate for changes in objective cognitive performance (attention, memory, and executive function) and psychiatric symptom severity (depression, anxiety, fatigue, and pain) in patients before, during and after interferon-alpha based therapy (IFN) for chronic hepatitis C virus infection (HCV).
Methods: 33 HCV+ adults were evaluated two months before IFN initiation (baseline), three months into IFN, and six months following IFN termination (IFN+ Group). 31 HCV+ adults who did not undergo IFN therapy were evaluated at baseline and six months later (IFN- Group). At each evaluation, participants completed the Neuropsychological Assessment Battery (NAB) Attention, Memory and Executive Functions Modules, the Beck Depression Inventory, Second Edition (BDI), Generalized Anxiety Disorder Inventory (GADI), Fatigue Severity Scale (FSS), and Brief Pain Inventory (BPI).
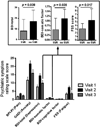
Results: Compared with the IFN- Group, the IFN+ Group experienced significantly (p<0.050) increased symptoms of depression, anxiety, fatigue and pain during IFN therapy relative to baseline. In the IFN+ Group, psychiatric symptoms generally returned to baseline levels following IFN termination. Sustained viral response was associated with significantly lower depression and fatigue. No significant changes in cognitive performance were observed.
Conclusions: During IFN, patients with HCV evidence significantly increased psychiatric symptoms, including symptoms of depression, anxiety, fatigue and pain. These psychiatric symptoms are generally short-term and remit following IFN termination, with increased benefit if viral clearance is achieved. However, IFN is not associated with significant declines in objective cognitive performance during or following IFN.
Keywords: Anxiety; Cognition; Depression; Fatigue; Hepatitis C; Interferon; Pain.
Copyright © 2014. Published by Elsevier Inc.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Similar articles
-
Mood, cognition and EEG changes during interferon alpha (alpha-IFN) treatment for chronic hepatitis C.J Affect Disord. 2005 Jan;84(1):93-8. doi: 10.1016/j.jad.2004.09.004. J Affect Disord. 2005. PMID: 15620390
-
Persistent neurocognitive decline in a clinic sample of hepatitis C virus-infected persons receiving interferon and ribavirin treatment.J Neurovirol. 2014 Dec;20(6):561-70. doi: 10.1007/s13365-014-0265-3. Epub 2014 Oct 18. J Neurovirol. 2014. PMID: 25326107 Free PMC article.
-
[Depressive symptoms during treatment with interferon alpha for HCV infection--preliminary report].Psychiatr Pol. 2006 Jul-Aug;40(4):799-808. Psychiatr Pol. 2006. PMID: 17068951 Polish.
-
Neuropsychiatric adverse effects of interferon-alpha: recognition and management.CNS Drugs. 2005;19(2):105-23. doi: 10.2165/00023210-200519020-00002. CNS Drugs. 2005. PMID: 15697325 Free PMC article. Review.
-
Hepatitis C treatment in patients with drug addiction: clinical management of interferon-alpha-associated psychiatric side effects.Curr Drug Abuse Rev. 2008 Jun;1(2):177-87. doi: 10.2174/1874473710801020177. Curr Drug Abuse Rev. 2008. PMID: 19630716 Review.
Cited by
-
Alcohol intake alters immune responses and promotes CNS viral persistence in mice.Behav Brain Res. 2016 Oct 1;312:1-8. doi: 10.1016/j.bbr.2016.06.006. Epub 2016 Jun 4. Behav Brain Res. 2016. PMID: 27269869 Free PMC article.
-
Hepatitis C virus eradication improves immediate and delayed episodic memory in patients treated with interferon and ribavirin.BMC Gastroenterol. 2017 Nov 25;17(1):122. doi: 10.1186/s12876-017-0679-5. BMC Gastroenterol. 2017. PMID: 29178838 Free PMC article.
-
Cost-effectiveness of Interferon-free therapy for Hepatitis C in Germany--an application of the efficiency frontier approach.BMC Infect Dis. 2015 Jul 30;15:297. doi: 10.1186/s12879-015-1048-z. BMC Infect Dis. 2015. PMID: 26223310 Free PMC article.
-
Assessment of factors associated with the quality of life of patients living with HIV/HCV co-infection.J Behav Med. 2016 Oct;39(5):767-81. doi: 10.1007/s10865-016-9778-y. Epub 2016 Aug 9. J Behav Med. 2016. PMID: 27506910
-
Anti-IFNAR treatment does not reverse neuropsychiatric disease in MRL/lpr lupus mice.Lupus. 2019 Nov;28(13):1510-1523. doi: 10.1177/0961203319872265. Epub 2019 Aug 31. Lupus. 2019. PMID: 31474191 Free PMC article.
References
-
- Global Burden Of Hepatitis CWG. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29. http://dx.doi.org/10.1177/0091270003258669 [PubMed PMID: 14681338]. - DOI - PubMed
-
- Seeff LB, Hoofnagle JH. Appendix: The National Institutes of Health Consensus Development Conference Management of Hepatitis C 2002. Clin Liver Dis. 2003;7:261–287. [PubMed PMID: 12691470]. - PubMed
-
- Yee HS, Chang MF, Pocha C, Lim J, Ross D, Morgan TR, et al. Update on the management and treatment of hepatitis C virus infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program Office. Am J Gastroenterol. 2012;107:669–689. http://dx.doi.org/10.1038/ajg.2012.48 [quiz 90, PubMed PMID: 22525303]. - DOI - PubMed
-
- Hauser P, Morasco BJ, Linke A, Bjornson D, Ruimy S, Matthews A, et al. Antiviral completion rates and sustained viral response in hepatitis C patients with and without preexisting major depressive disorder. Psychosomatics. 2009;50:500–505. http://dx.doi.org/10.1176/appi.psy.50.5.500 [PubMed PMID: 19855036; PubMed Central PMCID: PMC2987665]. - DOI - PMC - PubMed
-
- Chou R, Hartung D, Rahman B, Wasson N, Cottrell EB, Fu R. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: a systematic review. Ann Intern Med. 2013;158:114–123. [PubMed PMID: 23437439]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

