Hydrogen peroxide stimulation of CFTR reveals an Epac-mediated, soluble AC-dependent cAMP amplification pathway common to GPCR signalling
- PMID: 25220136
- PMCID: PMC4280976
- DOI: 10.1111/bph.12934
Hydrogen peroxide stimulation of CFTR reveals an Epac-mediated, soluble AC-dependent cAMP amplification pathway common to GPCR signalling
Abstract
Background and purpose: H2 O2 is widely understood to regulate intracellular signalling. In airway epithelia, H2 O2 stimulates anion secretion primarily by activating an autocrine PGE2 signalling pathway via EP4 and EP1 receptors to initiate cytic fibrosis transmembrane regulator (CFTR)-mediated Cl(-) secretion. This study investigated signalling downstream of the receptors activated by H2 O2 .
Experimental approach: Anion secretion by differentiated bronchial epithelial cells was measured in Ussing chambers during stimulation with H2 O2 , an EP4 receptor agonist or β2 -adrenoceptor agonist in the presence and absence of inhibitors of ACs and downstream effectors. Intracellular calcium ([Ca(2+) ]I ) changes were followed by microscopy using fura-2-loaded cells and PKA activation followed by FRET microscopy.
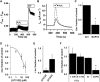
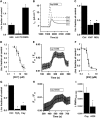
Key results: Transmembrane adenylyl cyclase (tmAC) and soluble AC (sAC) were both necessary for H2 O2 and EP4 receptor-mediated CFTR activation in bronchial epithelia. H2 O2 and EP4 receptor agonist stimulated tmAC to increase exchange protein activated by cAMP (Epac) activity that drives PLC activation to raise [Ca(2+) ]i via Ca(2+) store release (and not entry). Increased [Ca(2+) ]i led to sAC activation and further increases in CFTR activity. Stimulation of sAC did not depend on changes in [HCO3 (-) ]. Ca(2+) -activated apical KCa 1.1 channels and cAMP-activated basolateral KV 7.1 channels contributed to H2 O2 -stimulated anion currents. A similar Epac-mediated pathway was seen following β2 -adrenoceptor or forskolin stimulation.
Conclusions and implications: H2 O2 initiated a complex signalling cascade that used direct stimulation of tmACs by Gαs followed by Epac-mediated Ca(2+) crosstalk to activate sAC. The Epac-mediated Ca(2+) signal constituted a positive feedback loop that amplified CFTR anion secretion following stimulation of tmAC by a variety of stimuli.
© 2014 The British Pharmacological Society.
Figures





Similar articles
-
Amplification of FSH signalling by CFTR and nuclear soluble adenylyl cyclase in the ovary.Clin Exp Pharmacol Physiol. 2017 Dec;44 Suppl 1:78-85. doi: 10.1111/1440-1681.12756. Epub 2017 Sep 20. Clin Exp Pharmacol Physiol. 2017. PMID: 28345252 Review.
-
H2O2 stimulates cystic fibrosis transmembrane conductance regulator through an autocrine prostaglandin pathway, using multidrug-resistant protein-4.Am J Respir Cell Mol Biol. 2013 Oct;49(4):672-9. doi: 10.1165/rcmb.2013-0156OC. Am J Respir Cell Mol Biol. 2013. PMID: 23742099 Free PMC article.
-
CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures.Mol Biol Cell. 2010 Aug 1;21(15):2639-48. doi: 10.1091/mbc.E09-12-1004. Epub 2010 Jun 16. Mol Biol Cell. 2010. PMID: 20554763 Free PMC article.
-
Prostaglandin E2 induces chloride secretion through crosstalk between cAMP and calcium signaling in mouse inner medullary collecting duct cells.Am J Physiol Cell Physiol. 2014 Feb 1;306(3):C263-78. doi: 10.1152/ajpcell.00381.2012. Epub 2013 Nov 27. Am J Physiol Cell Physiol. 2014. PMID: 24284792 Free PMC article.
-
The secret life of CFTR as a calcium-activated chloride channel.J Physiol. 2013 Nov 1;591(21):5273-8. doi: 10.1113/jphysiol.2013.261909. Epub 2013 Aug 19. J Physiol. 2013. PMID: 23959675 Free PMC article. Review.
Cited by
-
Pharmacological modulation of the CO2/HCO3-/pH-, calcium-, and ATP-sensing soluble adenylyl cyclase.Pharmacol Ther. 2018 Oct;190:173-186. doi: 10.1016/j.pharmthera.2018.05.008. Epub 2018 May 26. Pharmacol Ther. 2018. PMID: 29807057 Free PMC article. Review.
-
Mammalian pigmentation is regulated by a distinct cAMP-dependent mechanism that controls melanosome pH.Sci Signal. 2018 Nov 6;11(555):eaau7987. doi: 10.1126/scisignal.aau7987. Sci Signal. 2018. PMID: 30401788 Free PMC article.
-
The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion.FASEB J. 2016 Mar;30(3):1144-54. doi: 10.1096/fj.15-277798. Epub 2015 Nov 17. FASEB J. 2016. PMID: 26578688 Free PMC article.
-
Biochemical pharmacology of adenylyl cyclases in cancer.Biochem Pharmacol. 2024 Oct;228:116160. doi: 10.1016/j.bcp.2024.116160. Epub 2024 Mar 24. Biochem Pharmacol. 2024. PMID: 38522554 Review.
-
Cigarette smoke activates CFTR through ROS-stimulated cAMP signaling in human bronchial epithelial cells.Am J Physiol Cell Physiol. 2018 Jan 1;314(1):C118-C134. doi: 10.1152/ajpcell.00099.2017. Epub 2017 Oct 4. Am J Physiol Cell Physiol. 2018. PMID: 28978522 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

