A new bispecific antibody targeting non-overlapping epitopes on IGF2: design, in vitro characterization and pharmacokinetics in macaques
- PMID: 25220345
- PMCID: PMC4262567
- DOI: 10.1016/j.yexmp.2014.09.007
A new bispecific antibody targeting non-overlapping epitopes on IGF2: design, in vitro characterization and pharmacokinetics in macaques
Abstract
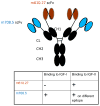
The insulin-like growth factor 2 (IGF2) is an important target for cancer therapy. We have previously proposed an approach for fast and irreversible removal of IGF2 from the circulation by using monoclonal antibodies (mAbs) that bind to two or more non-overlapping epitopes on the same molecule. We provided initial evidence for the formation of oligomeric antibody-ligand complexes that can bind to cells expressing Fc gamma receptors (FcγRs) with high avidity using an antibody domain with relatively low affinity as one of the anti-IGF2 mAbs. Recently, we identified a mAb, m708.5, in a scFv format which binds to both IGF2 and IGF1 with very high (pM) affinity. Interestingly, and rather surprisingly, this mAb did not compete with our other high affinity mAb, m610.27, for binding to IGF2. Therefore, we generated a new bispecific mAb, m67, by combining m708.5 and m610.27. As expected m67 potently inhibited binding of IGF2 to cells expressing the IGF1R and its phosphorylation, and resulted in formation of multimolecular complexes when incubated with IGF2 and bound with high avidity to cells expressing FcγRII; the complexes were internalized in a macrophage-like cell line. However, although m67 exhibited a reasonably long half-life (6.4 ± 0.6 days) in cynomolgus macaques and high stability in serum, its administration to three animals did not result in any measurable decrease in the IGF2 concentration likely due to the complexity of the IGF2 interactions in the blood and the relatively low (2mg/kg) dose of the mAb leading to a relatively low maximal blood concentration of 120nM. In spite of the lack of effect on the IGF2 concentration in this particular experimental setup, m67 exhibited good drugability properties and could be highly effective in other animal models and in humans. Studies with animal models of cancer are ongoing to evaluate the potential of m67 as a new candidate mAb-based therapeutic.
Keywords: Bispecific antibodies; Cynomolgus macaques; Half-life; IGF ligand.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures









Similar articles
-
Human monoclonal antibodies targeting nonoverlapping epitopes on insulin-like growth factor II as a novel type of candidate cancer therapeutics.Mol Cancer Ther. 2012 Jul;11(7):1400-10. doi: 10.1158/1535-7163.MCT-12-0172. Epub 2012 May 2. Mol Cancer Ther. 2012. PMID: 22553356 Free PMC article.
-
A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity.MAbs. 2011 May-Jun;3(3):273-88. doi: 10.4161/mabs.3.3.15188. Epub 2011 May 1. MAbs. 2011. PMID: 21393993 Free PMC article.
-
Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics.MAbs. 2014;6(5):1243-54. doi: 10.4161/mabs.29445. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517309 Free PMC article.
-
[Bispecific antibodies: what future?].Med Sci (Paris). 2009 Dec;25(12):1155-8. doi: 10.1051/medsci/200925121155. Med Sci (Paris). 2009. PMID: 20035697 Review. French.
-
Full-length recombinant antibodies from Escherichia coli: production, characterization, effector function (Fc) engineering, and clinical evaluation.MAbs. 2022 Jan-Dec;14(1):2111748. doi: 10.1080/19420862.2022.2111748. MAbs. 2022. PMID: 36018829 Free PMC article. Review.
Cited by
-
An insulin growth factor-I/II-neutralizing monoclonal antibody in combination with epidermal growth factor receptor inhibitors potently inhibits tumor cell growth.J Cancer. 2022 Mar 21;13(6):1830-1836. doi: 10.7150/jca.69064. eCollection 2022. J Cancer. 2022. PMID: 35399718 Free PMC article.
-
Tandem phage-display for the identification of non-overlapping binding pairs of recombinant affinity reagents.Nucleic Acids Res. 2017 Oct 13;45(18):e158. doi: 10.1093/nar/gkx688. Nucleic Acids Res. 2017. PMID: 28985360 Free PMC article.
-
Targeted Fcγ Receptor (FcγR)-mediated Clearance by a Biparatopic Bispecific Antibody.J Biol Chem. 2017 Mar 10;292(10):4361-4370. doi: 10.1074/jbc.M116.770628. Epub 2017 Jan 18. J Biol Chem. 2017. PMID: 28100773 Free PMC article.
-
Expanding the Boundaries of Biotherapeutics with Bispecific Antibodies.BioDrugs. 2018 Oct;32(5):441-464. doi: 10.1007/s40259-018-0299-9. BioDrugs. 2018. PMID: 30132211 Free PMC article. Review.
-
A dual-specific anti-IGF-1/IGF-2 human monoclonal antibody alone and in combination with temsirolimus for therapy of neuroblastoma.Int J Cancer. 2015 Nov 1;137(9):2243-52. doi: 10.1002/ijc.29588. Epub 2015 May 19. Int J Cancer. 2015. PMID: 25924852 Free PMC article.
References
-
- Corvaia N, et al. Insulin-like growth factor receptor type I as a target for cancer therapy. Front Biosci (Schol Ed) 2013;5:439–50. - PubMed
-
- Daughaday WH, et al. Inhibition of access of bound somatomedin to membrane receptor and immunobinding sites: a comparison of radioreceptor and radioimmunoassay of somatomedin in native and acid-ethanol-extracted serum. J Clin Endocrinol Metab. 1980;51:781–8. - PubMed
-
- Feng Y, Dimitrov DS. Insulin-like Growth Factors as Targets for Cancer Treatment. In: Malloy AH, Carson EC, editors. Oncogene Proteins: New Research. Nova Science Publishers; Hauppauge, New York: 2008a. pp. 161–176.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

