Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration
- PMID: 25220576
- PMCID: PMC4245375
- DOI: 10.1002/stem.1847
Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration
Abstract
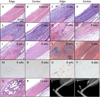
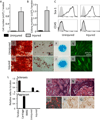
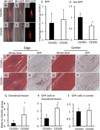
To study the cellular mechanism of the tendon repair process, we used a mouse Achilles tendon injury model to focus on the cells recruited to the injured site. The cells isolated from injured tendon 1 week after the surgery and uninjured tendons contained the connective tissue progenitor populations as determined by colony-forming capacity, cell surface markers, and multipotency. When the injured tendon-derived progenitor cells (inTPCs) were transplanted into injured Achilles tendons, they were not only integrated in the regenerating area expressing tenogenic phenotype but also trans-differentiated into chondrogenic cells in the degenerative lesion that underwent ectopic endochondral ossification. Surprisingly, the micromass culture of the inTPCs rapidly underwent chondrogenic differentiation even in the absence of exogenous bone morphogenetic proteins or TGFβs. The cells isolated from human ruptured tendon tissues also showed connective tissue progenitor properties and exhibited stronger chondrogenic ability than bone marrow stromal cells. The mouse inTPCs contained two subpopulations one positive and one negative for CD105, a coreceptor of the TGFβ superfamily. The CD105-negative cells showed superior chondrogenic potential in vitro and induced larger chondroid degenerative lesions in mice as compared to the CD105-positive cells. These findings indicate that tendon progenitor cells are recruited to the injured site of tendons and have a strong chondrogenic potential and that the CD105-negative population of these cells would be the cause for chondroid degeneration in injured tendons. The newly identified cells recruited to the injured tendon may provide novel targets to develop therapeutic strategies to facilitate tendon repair.
Keywords: Chondrogenesis; Injury; Progenitor; TGFβ; Tendon.
© 2014 AlphaMed Press.
Figures




References
-
- Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187–202. - PubMed
-
- Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford) 2006;45(5):508–521. - PubMed
-
- Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75(3):226–236. - PubMed
-
- Manske PR, Gelberman RH, Vande Berg JS, et al. Intrinsic flexor-tendon repair. A morphological study in vitro. J Bone Joint Surg Am. 1984;66(3):385–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

