Roles of plant hormones in the regulation of host-virus interactions
- PMID: 25220680
- PMCID: PMC6638471
- DOI: 10.1111/mpp.12204
Roles of plant hormones in the regulation of host-virus interactions
Abstract
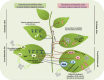
Hormones are tuners of plant responses to biotic and abiotic stresses. They are involved in various complicated networks, through which they modulate responses to different stimuli. Four hormones primarily regulate plant defence to pathogens: salicylic acid (SA), jasmonic acid (JA), ethylene (Et) and abscisic acid (ABA). In susceptible plants, viral infections result in hormonal disruption, which manifests as the simultaneous induction of several antagonistic hormones. However, these antagonistic hormones may exhibit some sequential accumulation in resistant lines. Virus propagation is usually restricted by the activation of the small interfering RNA (siRNA) antiviral machinery and/or SA signalling pathway. Several studies have investigated these two systems, using different model viruses. However, the roles of hormones other than SA, especially those with antagonistic properties, such as ABA, have been neglected. Increasing evidence indicates that hormones control components of the small RNA system, which regulates many processes (including the siRNA antiviral machinery and the microRNA system) at the transcriptional or post-transcriptional level. Consequently, cross-talk between the antagonistic SA and ABA pathways modulates plant responses at multiple levels. In this review, we summarize recent findings on the different roles of hormones in the regulation of plant-virus interactions, which are helping us to elucidate the fine tuning of viral and plant systems by hormones.
Keywords: defence pathways; host-virus interaction; plant hormones; plant virus.
© 2014 THE AUTHORS. MOLECULAR PLANT PATHOLOGY PUBLISHED BY JOHN WILEY & SONS LTD AND BSPP.
Figures

References
-
- Alamillo, J.M. , Saenz, P. and Garcia, J.A. (2006) Salicylic acid‐mediated and RNA‐silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco. Plant J. 48, 217–227. - PubMed
-
- Alazem, M. , Lin, K.Y. and Lin, N.S. (2014) The abscisic acid pathway has multifaceted effects on the accumulation of bamboo mosaic virus. Mol. Plant–Microbe Interact. 27, 177–189. - PubMed
-
- Aloni, R. , Langhans, M. , Aloni, E. , Dreieicher, E. and Ullrich, C.I. (2005) Root‐synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J. Exp. Bot. 56, 1535–1544. - PubMed
-
- Andrade, O. , Latorre, B.A. and Escaffi, O. (1981) Tomato mosaic‐virus associated with shoestring symptom in Chilean tomatoes. Plant Dis. 65, 761–762.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

