Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice
- PMID: 25220812
- PMCID: PMC4184914
- DOI: 10.1016/j.neuron.2014.08.037
Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice
Abstract
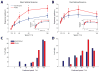
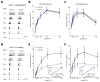
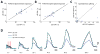
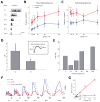
Neural circuits in the brain often receive inputs from multiple sources, such as the bottom-up input from early processing stages and the top-down input from higher-order areas. Here we study the function of top-down input in the mouse superior colliculus (SC), which receives convergent inputs from the retina and visual cortex. Neurons in the superficial SC display robust responses and speed tuning to looming stimuli that mimic approaching objects. The looming-evoked responses are reduced by almost half when the visual cortex is optogenetically silenced in awake, but not in anesthetized, mice. Silencing the cortex does not change the looming speed tuning of SC neurons, or the response time course, except at the lowest tested speed. Furthermore, the regulation of SC responses by the corticotectal input is organized retinotopically. This effect we revealed may thus provide a potential substrate for the cortex, an evolutionarily new structure, to modulate SC-mediated visual behaviors.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Comment in
-
A cortical rein on the tectum's gain.Neuron. 2014 Oct 1;84(1):6-8. doi: 10.1016/j.neuron.2014.09.021. Neuron. 2014. PMID: 25277449
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

