Mistranslation of the genetic code
- PMID: 25220850
- PMCID: PMC4254111
- DOI: 10.1016/j.febslet.2014.08.035
Mistranslation of the genetic code
Abstract
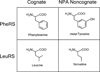
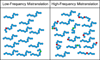
During mRNA decoding at the ribosome, deviations from stringent codon identity, or "mistranslation," are generally deleterious and infrequent. Observations of organisms that decode some codons ambiguously, and the discovery of a compensatory increase in mistranslation frequency to combat environmental stress have changed the way we view "errors" in decoding. Modern tools for the study of the frequency and phenotypic effects of mistranslation can provide quantitative and sensitive measurements of decoding errors that were previously inaccessible. Mistranslation with non-protein amino acids, in particular, is an enticing prospect for new drug therapies and the study of molecular evolution.
Keywords: Amino acid; Mistranslation; Protein synthesis; Quality control; Translation; tRNA.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
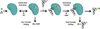
References
-
- Ibba M, Soll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. - PubMed
-
- Reynolds NM, Lazazzera BA, Ibba M. Cellular mechanisms that control mistranslation. Nat Rev Microbiol. 2010;8:849–856. - PubMed
-
- Lee JW, et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

