Role of FAM18B in diabetic retinopathy
- PMID: 25221423
- PMCID: PMC4124103
Role of FAM18B in diabetic retinopathy
Abstract
Purpose: Genome-wide association studies have suggested an association between a previously uncharacterized gene, FAM18B, and diabetic retinopathy. This study explores the role of FAM18B in diabetic retinopathy. An improved understanding of FAM18B could yield important insights into the pathogenesis of this sight-threatening complication of diabetes mellitus.
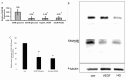
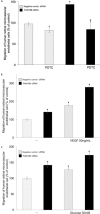
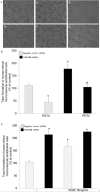
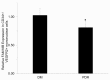
Methods: Postmortem human eyes were examined with immunohistochemistry and immunofluorescence for the presence of FAM18B. Expression of FAM18B in primary human retinal microvascular endothelial cells (HRMECs) exposed to hyperglycemia, vascular endothelial growth factor (VEGF), or advanced glycation end products (AGEs) was determined with quantitative reverse-transcription PCR (qRT-PCR) and/or western blot. The role of FAM18B in regulating human retinal microvascular endothelial cell viability, migration, and endothelial tube formation was determined following RNAi-mediated knockdown of FAM18B. The presence of FAM18B was determined with qRT-PCR in CD34+/VEGFR2+ mononuclear cells isolated from a cohort of 17 diabetic subjects with and without diabetic retinopathy.
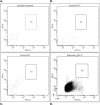
Results: Immunohistochemistry and immunofluorescence demonstrated the presence of FAM18B in the human retina with prominent vascular staining. Hyperglycemia, VEGF, and AGEs downregulated the expression of FAM18B in HRMECs. RNAi-mediated knockdown of FAM18B in HRMECs contributed to enhanced migration and tube formation as well as exacerbating the hyperglycemia-induced decrease in HRMEC viability. The enhanced migration, tube formation, and decrease in the viability of HRMECs as a result of FAM18B downregulation was reversed with pyrrolidine dithiocarbamate (PDTC), a specific nuclear factor-kappa B (NF-κB) inhibitor. CD34+/VEGFR2+ mononuclear cells from subjects with proliferative diabetic retinopathy demonstrated significantly reduced mRNA expression of FAM18B compared to diabetic subjects without retinopathy.
Conclusions: FAM18B is expressed in the retina. Diabetic culture conditions decrease the expression of FAM18B in HRMECs. The downregulation of FAM18B by siRNA in HRMECs results in enhanced migration and tube formation, but also exacerbates the hyperglycemia-induced decrease in HRMEC viability. The pathogenic changes observed in HRMECs as a result of FAM18B downregulation were reversed with PDTC, a specific NF-κB inhibitor. This study is the first to demonstrate a potential role for FAM18B in the pathogenesis of diabetic retinopathy.
Figures



References
-
- National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. 2011 [cited 2012 02/02/2012]; Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf
-
- Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–68. - PubMed
-
- Engerman RL, Kern TS. Hyperglycemia as a cause of diabetic retinopathy. Metabolism. 1986;35(Suppl 1):20–3. - PubMed
-
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

