Nutrient Sensing via mTOR in T Cells Maintains a Tolerogenic Microenvironment
- PMID: 25221554
- PMCID: PMC4147234
- DOI: 10.3389/fimmu.2014.00409
Nutrient Sensing via mTOR in T Cells Maintains a Tolerogenic Microenvironment
Abstract
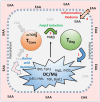
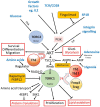
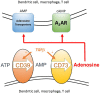
We have proposed that tolerance can be maintained through the induction, by Treg cells, of a tolerogenic microenvironment within tolerated tissues that inhibits effector cell activity but which supports the generation of further Treg cells by "infectious tolerance." Two important components of this tolerogenic microenvironment depend on metabolism and nutrient sensing. The first is due to the up-regulation of multiple enzymes that consume essential amino acids, which are sensed in naïve T cells primarily via inhibition of the mechanistic target of rapamycin (mTOR) pathway, which in turn encourages their further differentiation into FOXP3(+) Treg cells. The second mechanism is the metabolism of extracellular ATP to adenosine by the ectoenzymes CD39 and CD73. These two enzymes are constitutively co-expressed on Treg cells, but can also be induced on a wide variety of cell types by TGFβ and the adenosine generated can be shown to be a potent inhibitor of T cell proliferation. This review will focus on mechanisms of nutrient sensing in T cells, how these are integrated with TCR and cytokine signals via the mTOR pathway, and what impact this has on intracellular metabolism and subsequently the control of differentiation into different effector or regulatory T cell subsets.
Keywords: T cell differentiation; immune regulation; mTOR; metabolism; tolerance.
Figures


References
-
- Warburg O. On respiratory impairment in cancer cells. Science (1956) 124:269–70 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

