Cofactor engineering for enhancing the flux of metabolic pathways
- PMID: 25221776
- PMCID: PMC4147997
- DOI: 10.3389/fbioe.2014.00030
Cofactor engineering for enhancing the flux of metabolic pathways
Abstract
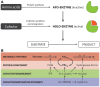
The manufacture of a diverse array of chemicals is now possible with biologically engineered strains, an approach that is greatly facilitated by the emergence of synthetic biology. This is principally achieved through pathway engineering in which enzyme activities are coordinated within a genetically amenable host to generate the product of interest. A great deal of attention is typically given to the quantitative levels of the enzymes with little regard to their overall qualitative states. This highly constrained approach fails to consider other factors that may be necessary for enzyme functionality. In particular, enzymes with physically bound cofactors, otherwise known as holoenzymes, require careful evaluation. Herein, we discuss the importance of cofactors for biocatalytic processes and show with empirical examples why the synthesis and integration of cofactors for the formation of holoenzymes warrant a great deal of attention within the context of pathway engineering.
Keywords: Fe–S clusters; cofactors; enzymatic activity; metabolic pathway engineering; synthetic biology.
Figures
References
-
- Broderick J. B. (2001). Coenzyme and Cofactors. eLS
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

