Brain Vascular Malformation Consortium: Overview, Progress and Future Directions
- PMID: 25221778
- PMCID: PMC4160161
Brain Vascular Malformation Consortium: Overview, Progress and Future Directions
Abstract
Brain vascular malformations are resource-intensive to manage effectively, are associated with serious neurological morbidity, lack specific medical therapies, and have no validated biomarkers for disease severity and progression. Investigators have tended to work in "research silos" with suboptimal cross-communication. We present here a paradigm for interdisciplinary collaboration to facilitate rare disease research. The Brain Vascular Malformation Consortium (BVMC) is a multidisciplinary, inter-institutional group of investigators, one of 17 consortia in the Office of Rare Disease Research Rare Disease Clinical Research Network (RDCRN). The diseases under study are: familial Cerebral Cavernous Malformations type 1, common Hispanic mutation (CCM1-CHM); Sturge-Weber Syndrome (SWS); and brain arteriovenous malformation in hereditary hemorrhagic telangiectasia (HHT). Each project is developing biomarkers for disease progression and severity, and has established scalable, relational databases for observational and longitudinal studies that are stored centrally by the RDCRN Data Management and Coordinating Center. Patient Support Organizations (PSOs) are a key RDCRN component in the recruitment and support of participants. The BVMC PSOs include Angioma Alliance, Sturge Weber Foundation, and HHT Foundation International. Our networks of clinical centers of excellence in SWS and HHT, as well as our PSOs, have enhanced BVMC patient recruitment. The BVMC provides unique and valuable resources to the clinical neurovascular community, and recently reported findings are reviewed. Future planned studies will apply successful approaches and insights across the three projects to leverage the combined resources of the BVMC and RDCRN in advancing new biomarkers and treatment strategies for patients with vascular malformations.
Figures
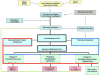
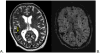
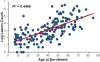
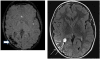

References
-
- Health Promotion and Disease Prevention Amendments of 1984. 1984 Oct 30; Pub. L. 98-551, 98 Stat. 2815.
-
- Orphan Drug Act of 1983. 1983 Jan 4; Pub. L. No. 97-414, 96 Stat. 2049.
-
- Gupta S, Bayoumi AM, Faughnan ME. Rare lung disease research: strategies for improving identification and recruitment of research participants. Chest. 2011;140:1123–1129. - PubMed
-
- Rare Diseases Clinical Research Network. BVMC: Brain Vascular Malformation Consortium. [July 1, 2012]; [Internet]. http://rarediseasesnetwork.epi.usf.edu/BVMC/index.htm.
-
- Office of Rare Diseases. Department of Health and Human Services, National Institutes of Health; 2001. [July 1, 2012]. Report on Steps to Coordinate Rare Diseases Research Programs: Analysis by and Recommendations of the Special Emphasis Panel of the National Institutes of Health on the Coordination of Rare Diseases Research. http://rarediseases.info.nih.gov/news-reports/fy99annual/SEP.html.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
