Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults
- PMID: 25221796
- PMCID: PMC4174344
- DOI: 10.1002/14651858.CD003256.pub2
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults
Abstract
Background: Kaposi's sarcoma remains the most common cancer in Sub-Saharan Africa and the second most common cancer in HIV-infected patients worldwide. Since the introduction of highly active antiretroviral therapy (HAART), there has been a decline in its incidence.However, Kaposi's sarcoma continues to be diagnosed in HIV-infected patients.
Objectives: To assess the added advantage of chemotherapy plus HAART compared to HAART alone; and the advantages of different chemotherapy regimens in HAART and HAART naive HIV infected adults with severe or progressive Kaposi's sarcoma.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and , GATEWAY, the WHO Clinical Trials Registry Platform and the US National Institutes of Health's ClinicalTrials.gov for ongoing trials and the Aegis archive of HIV/AIDS for conference abstracts. An updated search was conducted in July 2014.
Selection criteria: Randomised trials and observational studies evaluating the effects of any chemotherapeutic regimen in combination with HAART compared to HAART alone, chemotherapy versus HAART, and comparisons between different chemotherapy regimens.
Data collection and analysis: Two review authors assessed the studies independently and extracted outcome data.We used the risk ratio (RR) with a 95% confidence interval (CI) as the measure of effect.We did not conduct meta-analysis as none of the included trials assessed identical chemotherapy regimens.
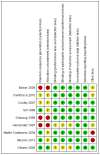
Main results: We included six randomised trials and three observational studies involving 792 HIV-infected adults with severe Kaposi's sarcoma.Seven studies included patients with a mix of mild to moderate (T0) and severe (T1) Kaposi's sarcoma. However, this review was restricted to the subset of participants with severe Kaposi's sarcoma disease.Studies comparing HAART plus chemotherapy to HAART alone showed the following: one trial comparing HAART plus doxorubicin,bleomycin and vincristine (ABV) to HAART alone showed a significant reduction in disease progression in the HAART plus ABV group (RR 0.10; 95% CI 0.01 to 0.75, 100 participants); there was no statistically significant reduction in mortality and no difference in adverse events. A cohort study comparing liposomal anthracyclines plus HAART to HAART alone showed a non-statistically significant reduction in Kaposi's sarcoma immune reconstitution inflammatory syndrome in patients that received HAART plus liposomal anthracyclines (RR 0.49; 95% CI 0.16 to 1.55, 129 participants).Studies comparing HAART plus chemotherapy to HAART plus a different chemotherapy regimen showed the following: one trial involving 49 participants and comparing paclitaxel versus pegylated liposomal doxorubicin in patients on HAART showed no difference in disease progression. Another trial involving 46 patients and comparing pegylated liposomal doxorubicin versus liposomal daunorubicin showed no participants with progressive Kaposi's sarcoma disease in either group.Studies comparing different chemotherapy regimens in patients from the pre-HAART era showed the following: in the single RCT comparing liposomal daunorubicin to ABV, there was no significant difference with the use of liposomal daunorubicin compared to ABV in disease progression (RR 0.78; 95% CI 0.34 to 1.82, 227 participants) and overall response rate. Another trial involving 178 participants and comparing oral etoposide versus ABV demonstrated no difference in mortality in either group. A non-randomised trial comparing bleomycin alone to ABV demonstrated a higher median survival time in the ABV group; there was also a non-statistically significant reduction in adverse events and disease progression in the ABV group (RR 11; 95% CI 0.67 to 179.29, 24 participants).An additional non-randomised study showed a non-statistically significant overall mortality benefit from liposomal doxorubicin as compared to conservative management consisting of either bleomycin plus vinblastine, vincristine or single-agent antiretroviral therapy alone (RR 0.93; 95% CI 0.75 to 1.15, 29 participants). The overall quality of evidence can be described as moderate quality. The quality of evidence was downgraded due to the small size of many of the included studies and small number of events.
Authors' conclusions: The findings from this review suggest that HAART plus chemotherapy may be beneficial in reducing disease progression compared to HAART alone in patients with severe or progressive Kaposi's sarcoma. For patients on HAART, when choosing from different chemotherapy regimens, there was no observed difference between liposomal doxorubicin, liposomal daunorubicin and paclitaxel.
Conflict of interest statement
Gbabe Oluwatoyin has no known conflict of interest.
Charles Okwundu has no known conflict of interest.
Martin Dedicoat has no known conflict of interest.
Esther Freeman has no known conflict of interest.
Figures
Update of
-
Treatment of Kaposi's sarcoma in HIV-1 infected individuals with emphasis on resource poor settings.Cochrane Database Syst Rev. 2003;(3):CD003256. doi: 10.1002/14651858.CD003256. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2014 Aug 13;(8):CD003256. doi: 10.1002/14651858.CD003256.pub2. PMID: 12917957 Updated.
References
References to studies included in this review
-
- Bower M, Dalla Pria A, Coyle C, Andrews E, Tittle V, Dhoot S, et al. Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. Journal of Clinical Oncology. 2014;32(5):409–14. - PubMed
-
- Bower M, Weir J, Francis N, Newsom-Davis T, Powles S, Crook T, et al. The effect of HAART in 254 consecutive patients with AIDS-related Kaposi's sarcoma. AIDS. 2009;23(13):1701–6. * Bower 2009 {published and unpublished data} - PubMed
-
- Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013;27(10):1603–13. PUBMED: 23462220. - PubMed
-
- Cianfrocca M, Lee S, Von Roenn J, Tulpule A, Dezube BJ, Aboulafia DM, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer. 2010;116(16):3969–77. [PUBMED: 20564162] Cianfrocca 2010 {published and unpublished data} - PMC - PubMed
-
- Cooley T, Henry D, Tonda M, Sun S, O’Connell M, Rackoff W. A randomized, double-blind study of pegylated liposomal doxorubicin for the treatment of AIDS-related Kaposi's sarcoma. The Oncologist. 2007;12(1):114–23. [PUBMED: 17227906] Cooley 2007 {published data only} - PubMed
References to studies excluded from this review
-
- Asiimwe F, Moore D, Were W, Nakityo R, Campbell J, Barasa A, et al. Clinical outcomes of HIV-infected patients with Kaposi's sarcoma receiving nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy in Uganda. HIV Medicine. 2012;13(3):166–71. [PUBMED: 22112164] Asiimwe 2012 {published data only} - PubMed
-
- Autier J, Picard-Dahan C, Marinho E, Grossin M, Yeni P, Leport C, et al. Docetaxel in anthracycline-pretreated AIDS-related Kaposi's sarcoma: a retrospective study. British Journal of Dermatology. 2005;152(5):1026–9. [PUBMED: 15888164] Autier 2005 {published data only} - PubMed
-
- Bihl F, Mosam A, Henry LN, Chisholm JV, 3rd, Dollard S, Gumbi P, et al. Kaposi's sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi's sarcoma. AIDS (London, England) 2007;21(10):1245. [PUBMED: 17545700] Bihl 2007 {published data only} - PubMed
-
- Bodsworth NJ, Bloch M, Donnell D, Yocum R, The International Panretin Gel KS Study Group Phase III vehicle controlled multi-centered study of topical alitretinoin gel 0.1% in cutaneous AIDS related Kaposi's sarcoma. American Journal of Clinical Dermatology. 2001;2(2):77–87. Bodsworth 2001 {published data only} - PubMed
-
- Bonhomme L, Fredj G, Averous S, Szekely AM, Ecstein E, Trumbic B, et al. Topical treatment of epidemic Kaposi's sarcoma with all-trans-retinoic acid. Annals of Oncology. 1991;2(3):234–5. [PUBMED: 2043496] Bonhomme 1991 {published data only} - PubMed
Additional references
-
- Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Current Opinion in Infectious Diseases. 2006;19(1):14–9. Bower 2006. - PubMed
-
- Browning PJ, Sechler JM, Kaplan M, Washington RH, Gendelman R, Yarchoan R, et al. Identification and culture of Kaposi's sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood. 1994;84:2711–20. Browning 1994. - PubMed
-
- Carrieri MP, Pradier C, Piselli P, Piche M, Rosenthal E, Heudier P, et al. Reduced incidence of Kaposi's sarcoma and of systemic non-Hodgkin's lymphoma in HIV-infected individuals treated with highly active antiretroviral therapy. International Journal of Cancer. 2003;103(1):142–4. Carrieri 2003. - PubMed
-
- Casper C. The increasing burden of HIV-associated malignancies in resource-limited regions. Annual Review of Medicine. 2011;62:157–70. Casper 2011. - PubMed
-
- Chang Y, Cesarman E, Pessin M, Lee F, Culpepper J, Knowles DM, et al. Identification of new human herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266(5192):1865–9. Chang 1994. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical