Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium
- PMID: 25222024
- PMCID: PMC4164635
- DOI: 10.1371/journal.pone.0107814
Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium
Abstract
Background: We previously reported in vitro maintenance and proliferation of human small intestinal epithelium using Matrigel, a proprietary basement membrane product. There are concerns over the applicability of Matrigel-based methods for future human therapies. We investigated type I collagen as an alternative for the culture of human intestinal epithelial cells.
Methods: Human small intestine was procured from fresh surgical pathology specimens. Small intestinal crypts were isolated using EDTA chelation. Intestinal subepithelial myofibroblasts were isolated from a pediatric sample and expanded in vitro. After suspension in Matrigel or type I collagen gel, crypts were co-cultured above a confluent layer of myofibroblasts. Crypts were also grown in monoculture with exposure to myofibroblast conditioned media; these were subsequently sub-cultured in vitro and expanded with a 1∶2 split ratio. Cultures were assessed with light microscopy, RT-PCR, histology, and immunohistochemistry.
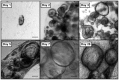
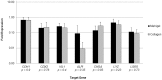
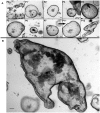
Results: Collagen supported viable human epithelium in vitro for at least one month in primary culture. Sub-cultured epithelium expanded through 12 passages over 60 days. Histologic sections revealed polarized columnar cells, with apical brush borders and basolaterally located nuclei. Collagen-based cultures gave rise to monolayer epithelial sheets at the gel-liquid interface, which were not observed with Matrigel. Immunohistochemical staining identified markers of differentiated intestinal epithelium and myofibroblasts. RT-PCR demonstrated expression of α-smooth muscle actin and vimentin in myofibroblasts and E-Cadherin, CDX2, villin 1, intestinal alkaline phosphatase, chromogranin A, lysozyme, and Lgr5 in epithelial cells. These markers were maintained through several passages.
Conclusion: Type I collagen gel supports long-term in vitro maintenance and expansion of fully elaborated human intestinal epithelium. Collagen-based methods yield familiar enteroid structures as well as a new pattern of sheet-like growth, and they eliminate the need for Matrigel for in vitro human intestinal epithelial growth. Future research is required to further develop this cell culture system for tissue engineering applications.
Conflict of interest statement
Figures





Similar articles
-
Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium.Tissue Eng Part C Methods. 2013 Dec;19(12):961-9. doi: 10.1089/ten.TEC.2012.0710. Epub 2013 May 10. Tissue Eng Part C Methods. 2013. PMID: 23566043 Free PMC article.
-
Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium.PLoS One. 2011;6(11):e26898. doi: 10.1371/journal.pone.0026898. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22125602 Free PMC article.
-
Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix.Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7717-22. doi: 10.1073/pnas.93.15.7717. Proc Natl Acad Sci U S A. 1996. PMID: 8755542 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Matrigel: from discovery and ECM mimicry to assays and models for cancer research.Adv Drug Deliv Rev. 2014 Dec 15;79-80:3-18. doi: 10.1016/j.addr.2014.06.005. Epub 2014 Jul 2. Adv Drug Deliv Rev. 2014. PMID: 24997339 Review.
Cited by
-
Recent advances in lung organoid development and applications in disease modeling.J Clin Invest. 2023 Nov 15;133(22):e170500. doi: 10.1172/JCI170500. J Clin Invest. 2023. PMID: 37966116 Free PMC article. Review.
-
Advances in Organoid Culture Research.Glob Med Genet. 2022 Dec 14;9(4):268-276. doi: 10.1055/s-0042-1756662. eCollection 2022 Dec. Glob Med Genet. 2022. PMID: 36530528 Free PMC article. Review.
-
Copper Transport and Disease: What Can We Learn from Organoids?Annu Rev Nutr. 2019 Aug 21;39:75-94. doi: 10.1146/annurev-nutr-082018-124242. Epub 2019 May 31. Annu Rev Nutr. 2019. PMID: 31150593 Free PMC article. Review.
-
Developments in gastrointestinal organoid cultures to recapitulate tissue environments.Front Bioeng Biotechnol. 2025 Apr 17;13:1521044. doi: 10.3389/fbioe.2025.1521044. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40313639 Free PMC article. Review.
-
Listen to Your Gut: Key Concepts for Bioengineering Advanced Models of the Intestine.Adv Sci (Weinh). 2024 Feb;11(5):e2302165. doi: 10.1002/advs.202302165. Epub 2023 Nov 27. Adv Sci (Weinh). 2024. PMID: 38009508 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

