Scientists versus regulators: precaution, novelty & regulatory oversight as predictors of perceived risks of engineered nanomaterials
- PMID: 25222742
- PMCID: PMC4164444
- DOI: 10.1371/journal.pone.0106365
Scientists versus regulators: precaution, novelty & regulatory oversight as predictors of perceived risks of engineered nanomaterials
Abstract
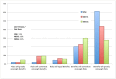
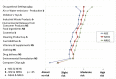
Engineered nanoscale materials (ENMs) present a difficult challenge for risk assessors and regulators. Continuing uncertainty about the potential risks of ENMs means that expert opinion will play an important role in the design of policies to minimize harmful implications while supporting innovation. This research aims to shed light on the views of 'nano experts' to understand which nanomaterials or applications are regarded as more risky than others, to characterize the differences in risk perceptions between expert groups, and to evaluate the factors that drive these perceptions. Our analysis draws from a web-survey (N = 404) of three groups of US and Canadian experts: nano-scientists and engineers, nano-environmental health and safety scientists, and regulatory scientists and decision-makers. Significant differences in risk perceptions were found across expert groups; differences found to be driven by underlying attitudes and perceptions characteristic of each group. Nano-scientists and engineers at the upstream end of the nanomaterial life cycle perceived the lowest levels of risk, while those who are responsible for assessing and regulating risks at the downstream end perceived the greatest risk. Perceived novelty of nanomaterial risks, differing preferences for regulation (i.e. the use of precaution versus voluntary or market-based approaches), and perceptions of the risk of technologies in general predicted variation in experts' judgments of nanotechnology risks. Our findings underscore the importance of involving a diverse selection of experts, particularly those with expertise at different stages along the nanomaterial lifecycle, during policy development.
Conflict of interest statement
Figures



References
-
- Kandlikar M, Ramachandran G, Maynard A, Murdock B (2007) Health risk assessment for nanoparticles: A case for using expert judgment. J Nanopart Res 9: 137–156.
-
- Presidents Council of Advisors on Science and Technology PCAST (2012) Report to the President and Congress on The Fourth Assessment of the National Nanotechnology Initiative. Washington, D.C. Available: http://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST_2012....
-
- Renn O, Roco MC (2006) Nanotechnology and the need for risk governance. J Nanopart Res 8: 153–191.
-
- Bosso CJ, editor (2010) Governing uncertainty: Environmental regulation in the age of nanotechnology. Routledge.
-
- Powell MC (2007) New risk or old risk, high risk or no risk? How scientists' standpoints shape their nanotechnology risk frames. Hlth, Risk & Soc 9: 173–190 10.1080/13698570701306872 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

