Blood pressure-lowering peptides from neo-fermented buckwheat sprouts: a new approach to estimating ACE-inhibitory activity
- PMID: 25222748
- PMCID: PMC4164440
- DOI: 10.1371/journal.pone.0105802
Blood pressure-lowering peptides from neo-fermented buckwheat sprouts: a new approach to estimating ACE-inhibitory activity
Abstract
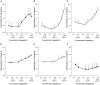
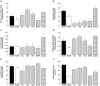
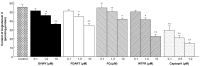
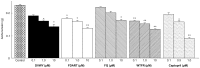
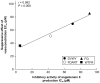
Neo-fermented buckwheat sprouts (neo-FBS) contain angiotensin-converting enzyme (ACE) inhibitors and vasodilators with blood pressure-lowering (BPL) properties in spontaneously hypertensive rats (SHRs). In this study, we investigated antihypertensive mechanisms of six BPL peptides isolated from neo-FBS (FBPs) by a vasorelaxation assay and conventional in vitro, in vivo, and a new ex vivo ACE inhibitory assays. Some FBPs demonstrated moderate endothelium-dependent vasorelaxation in SHR thoracic aorta and all FBPs mildly inhibited ACE in vitro. Orally administered FBPs strongly inhibited ACE in SHR tissues. To investigate detailed ACE-inhibitory mechanism of FBPs in living body tissues, we performed the ex vivo assay by using endothelium-denuded thoracic aorta rings isolated from SHRs, which demonstrated that FBPs at low concentration effectively inhibited ACE in thoracic aorta tissue and suppressed angiotensin II-mediated vasoconstriction directly associated with BPL. These results indicate that the main BPL mechanism of FBP was ACE inhibition in living body tissues, suggesting that high FBP's bioavailability including absorption, tissue affinity, and tissue accumulation was responsible for the superior ACE inhibition in vivo. We propose that our ex vivo assay is an efficient and reliable method for evaluating ACE-inhibitory mechanism responsible for BPL activity in vivo.
Conflict of interest statement
Figures
References
-
- Heeneman S, Sluimer JC, Daemen MJ (2007) Angiotensin-converting enzyme and vascular remodeling. Circ Res 101: 441–454. - PubMed
-
- Falkenhahn M, Franke F, Bohle RM, Zhu YC, Stauss HM, et al. (1995) Cellular distribution of angiotensin-converting enzyme after myocardial infarction. Hypertension 25: 219–226. - PubMed
-
- Fukuhara M, Geary RL, Diz DI, Gallagher PE, Wilson JA, et al. (2000) Angiotensin-converting enzyme expression in human carotid artery atherosclerosis. Hypertension 35: 353–359. - PubMed
-
- Fukuda N, Hu WY, Satoh C, Nakayama M, Kishioka H, et al. (1999) Contribution of synthetic phenotype on the enhanced angiotensin II-generating system in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens 17: 1099–1107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

