Immediate rescue designs in pediatric analgesic trials: a systematic review and meta-analysis
- PMID: 25222831
- PMCID: PMC4305029
- DOI: 10.1097/ALN.0000000000000445
Immediate rescue designs in pediatric analgesic trials: a systematic review and meta-analysis
Abstract
Background: Designing analgesic clinical trials in pediatrics requires a balance between scientific, ethical, and practical concerns. A previous consensus group recommended immediate rescue designs using opioid sparing as a surrogate measure of analgesic efficacy. The authors summarize the performance of rescue analgesic designs in pediatric trials of four commonly used classes of analgesics: opioids, nonsteroidal antiinflammatory drugs, acetaminophen, and local anesthetics.
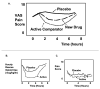
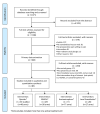
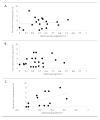
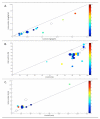
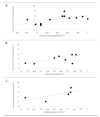
Methods: MEDLINE, Embase, CINAHL, The Cochrane Library, and Web of science were searched in April 2013. The 85 studies selected were randomized or controlled clinical trials using immediate rescue paradigms in postoperative pain settings. A random-effects meta-analysis was used to synthesize predefined outcomes using Hedges' g. Difference between the means of the treatment arms were also expressed as a percentage of the corresponding value in the placebo group (placebo-treatment/placebo). Distributions of pain scores in study and control groups and relationships between opioid sparing and pain scores were examined.
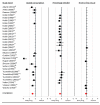
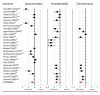
Results: For each of the four study drug classes, significant opioid sparing was demonstrated in a majority of studies by one or more of the following endpoints: (1) total dose (milligram per kilogram per hour), (2) percentage of children requiring rescue medication, and (3) time to first rescue medication (minutes). Pain scores averaged 2.4/10 in study groups, 3.4/10 in control groups.
Conclusions: Opioid sparing is a feasible pragmatic endpoint for pediatric pain analgesic trials. This review serves to guide future research in pediatric analgesia trials, which could test whether some specific design features may improve assay sensitivity while minimizing the risk of unrelieved pain.
Figures



References
-
- Berde CB, Walco GA, Krane EJ, Anand KJ, Aranda JV, Craig KD, Dampier CD, Finkel JC, Grabois M, Johnston C, Lantos J, Lebel A, Maxwell LG, McGrath P, Oberlander TF, Schanberg LE, Stevens B, Taddio A, von Baeyer CL, Yaster M, Zempsky WT. Pediatric analgesic clinical trial designs, measures, and extrapolation: Report of an FDA scientific workshop. Pediatrics. 2012;129:354–64. - PMC - PubMed
-
- Finkel JC, Finley A, Greco C, Weisman SJ, Zeltzer L. Transdermal fentanyl in the management of children with chronic severe pain: Results from an international study. Cancer. 2005;104:2847–57. - PubMed
-
- Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 2010;126:e1168–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

