Biomechanics of Schlemm's canal endothelium and intraocular pressure reduction
- PMID: 25223880
- PMCID: PMC4268318
- DOI: 10.1016/j.preteyeres.2014.08.002
Biomechanics of Schlemm's canal endothelium and intraocular pressure reduction
Abstract
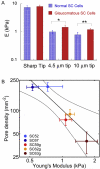
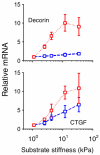
Ocular hypertension in glaucoma develops due to age-related cellular dysfunction in the conventional outflow tract, resulting in increased resistance to aqueous humor outflow. Two cell types, trabecular meshwork (TM) and Schlemm's canal (SC) endothelia, interact in the juxtacanalicular tissue (JCT) region of the conventional outflow tract to regulate outflow resistance. Unlike endothelial cells lining the systemic vasculature, endothelial cells lining the inner wall of SC support a transcellular pressure gradient in the basal to apical direction, thus acting to push the cells off their basal lamina. The resulting biomechanical strain in SC cells is quite large and is likely to be an important determinant of endothelial barrier function, outflow resistance and intraocular pressure. This review summarizes recent work demonstrating how biomechanical properties of SC cells impact glaucoma. SC cells are highly contractile, and such contraction greatly increases cell stiffness. Elevated cell stiffness in glaucoma may reduce the strain experienced by SC cells, decrease the propensity of SC cells to form pores, and thus impair the egress of aqueous humor from the eye. Furthermore, SC cells are sensitive to the stiffness of their local mechanical microenvironment, altering their own cell stiffness and modulating gene expression in response. Significantly, glaucomatous SC cells appear to be hyper-responsive to substrate stiffness. Thus, evidence suggests that targeting the material properties of SC cells will have therapeutic benefits for lowering intraocular pressure in glaucoma.
Keywords: Aqueous humor; Conventional outflow; Glaucoma; Ocular hypertension; Schlemm's canal; Trabecular meshwork.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures







References
-
- Allingham RR, de Kater AW, Ethier CR. Schlemm's canal and primary open angle glaucoma: correlation between Schlemm's canal dimensions and outflow facility. Exp. Eye Res. 1996;62:101–109. - PubMed
-
- Allingham RR, Dekater AW, Ethier CR, Anderson PJ, Hertzmark E, Epstein DL. The relationship between pore density and outflow facility in human eyes. Invest. Ophthalmol. Vis. Sci. 1992;33:1661–1669. - PubMed
-
- Alvarado J, Betanzos A, Franse-Carman L, Chen J, Gonzalez-Mariscal L. Endothelia of Schlemm's canal and trabecular meshwork: distinct molecular, functional, and anatomic features. Am. J. Physiol. 2004;286:C621–C634. - PubMed
-
- Alvarado J, Murphey C, Polanski J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest. Ophthalmol. Vis. Sci. 1981;21:714–727. - PubMed
-
- Alvarado J, Murphey C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Invest. Ophthalmol. Vis. Sci. 1984;91:564–579. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

