Induced pluripotent stem cells from ALS patients for disease modeling
- PMID: 25223906
- PMCID: PMC4362997
- DOI: 10.1016/j.brainres.2014.09.017
Induced pluripotent stem cells from ALS patients for disease modeling
Abstract
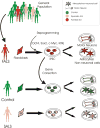
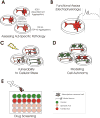
The ability to reprogram adult somatic cells into pluripotent stem cells that can differentiate into all three germ layers of the developing human has fundamentally changed the landscape of biomedical research. For a neurodegenerative disease like Amyotrophic Lateral Sclerosis (ALS), which does not manifest itself until adulthood and is a heterogeneous disease with few animal models, this technology may be particularly important. Induced pluripotent stem cells (iPSC) have been created from patients with several familial forms of ALS as well as some sporadic forms of ALS. These cells have been differentiated into ALS-relevant cell subtypes including motor neurons and astrocytes, among others. ALS-relevant pathologies have also been identified in motor neurons from these cells and may provide a window into understanding disease mechanisms in vitro. Given that this is a relatively new field of research, numerous challenges remain before iPSC methodologies can fulfill their potential as tools for modeling ALS as well as providing a platform for the investigation of ALS therapeutics. This article is part of a Special Issue entitled ALS complex pathogenesis.
Keywords: Astrocyte; Human; Motor neuron; Non-cell autonomous; Stem cell; iPSC.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
References
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, et al. Modeling key pathological features of frontotemporal dementia with C90RF72 repeat expansion in iPSC-derived human neurons. Acta neuropathologica. 2013;126:385–399. - PMC - PubMed
-
- Amoroso MW, Croft GF, Williams DJ, O'Keeffe S, Carrasco MA, Davis AR, Roybon L, Oakley DH, Maniatis T, Henderson CE, et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:574–586. - PMC - PubMed
-
- Benatar M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. NeurobiolDis. 2007;26:1–13. - PubMed
-
- Bilican B, Serio A, Barmada SJ, Nishimura AL, Sullivan GJ, Carrasco M, Phatnani HP, Puddifoot CA, Story D, Fletcher J, et al. Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5803–5808. - PMC - PubMed
-
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

