Population pharmacokinetics of teicoplanin in children
- PMID: 25224001
- PMCID: PMC4249354
- DOI: 10.1128/AAC.03685-14
Population pharmacokinetics of teicoplanin in children
Abstract
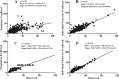
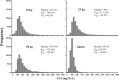
Teicoplanin is frequently administered to treat Gram-positive infections in pediatric patients. However, not enough is known about the pharmacokinetics (PK) of teicoplanin in children to justify the optimal dosing regimen. The aim of this study was to determine the population PK of teicoplanin in children and evaluate the current dosage regimens. A PK hospital-based study was conducted. Current dosage recommendations were used for children up to 16 years of age. Thirty-nine children were recruited. Serum samples were collected at the first dose interval (1, 3, 6, and 24 h) and at steady state. A standard 2-compartment PK model was developed, followed by structural models that incorporated weight. Weight was allowed to affect clearance (CL) using linear and allometric scaling terms. The linear model best accounted for the observed data and was subsequently chosen for Monte Carlo simulations. The PK parameter medians/means (standard deviation [SD]) were as follows: CL, [0.019/0.023 (0.01)] × weight liters/h/kg of body weight; volume, 2.282/4.138 liters (4.14 liters); first-order rate constant from the central to peripheral compartment (Kcp), 0.474/3.876 h(-1) (8.16 h(-1)); and first-order rate constant from peripheral to central compartment (Kpc), 0.292/3.994 h(-1) (8.93 h(-1)). The percentage of patients with a minimum concentration of drug in serum (Cmin) of <10 mg/liter was 53.85%. The median/mean (SD) total population area under the concentration-time curve (AUC) was 619/527.05 mg · h/liter (166.03 mg · h/liter). Based on Monte Carlo simulations, only 30.04% (median AUC, 507.04 mg · h/liter), 44.88% (494.1 mg · h/liter), and 60.54% (452.03 mg · h/liter) of patients weighing 50, 25, and 10 kg, respectively, attained trough concentrations of >10 mg/liter by day 4 of treatment. The teicoplanin population PK is highly variable in children, with a wider AUC distribution spread than for adults. Therapeutic drug monitoring should be a routine requirement to minimize suboptimal concentrations. (This trial has been registered in the European Clinical Trials Database Registry [EudraCT] under registration number 2012-005738-12.).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Verstraete E, Boelens J, De Coen K, Claeys G, Vogelaers D, Vanhaesebrouck P, Blot S. 2014. Healthcare-associated bloodstream infections in a neonatal intensive care unit over a 20-year period (1992–2011): trends in incidence, pathogens, and mortality. Infect. Control Hosp. Epidemiol. 35:511–518. 10.1086/675836. - DOI - PubMed
-
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45. 10.1086/599376. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

