Impact of antibiotics with various target sites on the metabolome of Staphylococcus aureus
- PMID: 25224006
- PMCID: PMC4249544
- DOI: 10.1128/AAC.03104-14
Impact of antibiotics with various target sites on the metabolome of Staphylococcus aureus
Abstract
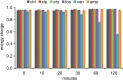
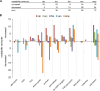
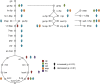
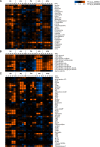
In this study, global intra- and extracellular metabolic profiles were exploited to investigate the impact of antibiotic compounds with different cellular targets on the metabolome of Staphylococcus aureus HG001. Primary metabolism was largely covered, yet uncommon staphylococcal metabolites were detected in the cytosol of S. aureus, including sedoheptulose-1,7-bisphosphate and the UDP-MurNAc-pentapeptide with an alanine-seryl residue. By comparing the metabolic profiles of unstressed and stressed staphylococcal cells in a time-dependent manner, we found far-ranging effects within the metabolome. For each antibiotic compound, accumulation as well as depletion of metabolites was detected, often comprising whole biosynthetic pathways, such as central carbon and amino acid metabolism and peptidoglycan, purine, and pyrimidine synthesis. Ciprofloxacin altered the pool of (deoxy)nucleotides as well as peptidoglycan precursors, thus linking stalled DNA and cell wall synthesis. Erythromycin tended to increase the amounts of intermediates of the pentose phosphate pathway and lysine. Fosfomycin inhibited the first enzymatic step of peptidoglycan synthesis, which was followed by decreased levels of peptidoglycan precursors but enhanced levels of substrates such as UDP-GlcNAc and alanine-alanine. In contrast, vancomycin and ampicillin inhibited the last stage of peptidoglycan construction on the outer cell surface. As a result, the amounts of UDP-MurNAc-peptides drastically increased, resulting in morphological alterations in the septal region and in an overall decrease in central metabolite levels. Moreover, each antibiotic affected intracellular levels of tricarboxylic acid cycle intermediates.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Utaida S, Dunman PM, Macapagal D, Murphy E, Projan SJ, Singh VK, Jayaswal RK, Wilkinson BJ. 2003. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149:2719–2732. 10.1099/mic.0.26426-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

