Pre-formulation studies of resveratrol
- PMID: 25224342
- PMCID: PMC4427559
- DOI: 10.3109/03639045.2014.958753
Pre-formulation studies of resveratrol
Abstract
Context: Resveratrol, a natural compound found in grapes, has potential chemotherapy effects but very low oral bioavailability in humans.
Objective: To evaluate the solubility, pH stability profile, plasma protein binding (PPB) and stability in plasma for resveratrol.
Methods: Solubility of resveratrol was measured in 10 common solvents at 25 °C using HPLC. The solution state pH stability of resveratrol was assessed in various United States Pharmacopeia buffers ranging from pH 2 to 10 for 24 h at 37 °C. Samples were analyzed up to 24 h. Human PPB was determined using ultracentrifugation technique. Standard solutions of drug were spiked to blank human plasma to yield final concentrations of 5, 12.5 or 25 μg/mL for determination. Finally, stability of resveratrol in human and rat plasma was also assessed at 37 °C. Aliquots of blank plasma were spiked with a standard drug concentration to yield final plasma concentration of 50 μg/mL. Samples were analyzed for resveratrol concentration up to 96 h.
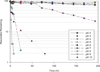
Results: Resveratrol has wide solubility ranging from 0.05 mg/mL in water to 374 mg/mL in polyethylene glycol 400 (PEG-400). Resveratrol is relatively stable above pH 6 and has maximum degradation at pH 9. The mean PPB of resveratrol is 98.3%. Resveratrol degrades in human and rat plasma in a first-order process with mean half lives of 54 and 25 h, respectively.
Conclusion: Resveratrol is more soluble in alcohol and PEG-400 and stable in acidic pH. It binds highly to plasma proteins and degrades slower in human then rat plasma.
Keywords: Natural compound; physiochemical evaluation; plasma protein binding; polyphenol; solubility; stability.
Figures





References
-
- Das S, Lin HS, Ho PC, Ng KY. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm Res. 2008;25:2594–2600. - PubMed
-
- Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953–957. - PubMed
-
- He H, Chen X, Wang G, et al. High-performanc liquid chromatography spectrometric analysis of trans-resveratrol in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832:177–180. - PubMed
-
- Juan ME, Lamuela-Raventós RM, de la Torre-Boronat MC, Planas JM. Determination of trans-resveratrol in plasma by HPLC. Anal Chem. 1999;71:747–750. - PubMed
-
- Delmas D, Lancon A, Colin D, et al. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
