Tacrine sinusoidal uptake and biliary excretion in sandwich-cultured primary rat hepatocytes
- PMID: 25224352
- PMCID: PMC7928264
- DOI: 10.18433/j3801t
Tacrine sinusoidal uptake and biliary excretion in sandwich-cultured primary rat hepatocytes
Abstract
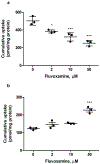
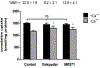
PURPOSE. The knowledge of hepatic disposition kinetics of tacrine, a first cholinesterase inhibitor was approved by FDA for the treatment of Alzheimer's disease (AD), would help to understand its hepatotoxicity, its therapeutic effect, and improve the management of patients with AD. The current study aims to characterize tacrine hepatic transport kinetics and study the role of organic cation transporters (OCTs), P-glycoprotein (P-gp) and multidrug resistance-associated protein (MRP2) in tacrine sinusoidal uptake and biliary excretion. METHODS. Modulation of tacrine hepatic uptake and efflux, biliary excretion index (BEI%), were performed in sandwich-cultured primary rat hepatocytes (SCHs) using transporters inhibitors. Conformation of the integrity of SCHs model was established by capturing images with light-contrast and fluorescence microscopy. RESULTS. Tacrine uptake in SCHs was carrier-mediated process and saturable with apparent Km of 31.5±9.6 µM and Vmax of 908±72 pmol/min/mg protein. Tetraethyl ammonium (TEA), cimetidine and verapamil significantly reduced tacrine uptake with more pronounced effect observed with verapamil which caused 3-fold reduction in tacrine uptake, indicating role for OCTs. Tacrine has a biliary excretion in SCHs with maximum BEI% value of 22.9±1.9% at 10 min of incubation. Addition of MK571 and valspodar decreased the BEI% of tacrine by 40 and 60% suggesting roles for canalicular MRP2 and P-gp, respectively. CONCLUSIONS. Our results show that in addition to metabolism, tacrine hepatic disposition is carrier-mediated process mediated by sinusoidal OCTs, and canalicular MRP2 and P-gp.
Figures




References
-
- Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging, 2004; 21(7):453–78. - PubMed
-
- Crismon ML. Tacrine: first drug approved for Alzheimer’s disease. Ann Pharmacother, 1994; 28(6):744–51. - PubMed
-
- Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J. A controlled trial of tacrine in Alzheimer’s disease. The Tacrine Study Group. JAMA, 1992; 268(18):2523–9. - PubMed
-
- Korabecny J, Spilovska K, Benek O, Musilek K, Soukup O, Kuca K. [Tacrine and its derivatives in the therapy of Alzheimers disease]. Ceska Slov Farm, 2012; 61(5):210–21. - PubMed
-
- Arrieta JL, Artalejo FR. Methodology, results and quality of clinical trials of tacrine in the treatment of Alzheimer’s disease: a systematic review of the literature. Age Ageing, 1998; 27(2):161–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

