α-1-Antitrypsin (AAT)-modified donor cells suppress GVHD but enhance the GVL effect: a role for mitochondrial bioenergetics
- PMID: 25224412
- PMCID: PMC4215316
- DOI: 10.1182/blood-2014-04-570440
α-1-Antitrypsin (AAT)-modified donor cells suppress GVHD but enhance the GVL effect: a role for mitochondrial bioenergetics
Abstract
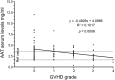
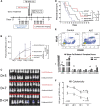
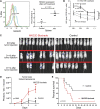
Hematopoietic cell transplantation is curative in many patients. However, graft-versus-host disease (GVHD), triggered by alloreactive donor cells, has remained a major complication. Here, we show an inverse correlation between plasma α-1-antitrypsin (AAT) levels in human donors and the development of acute GVHD in the recipients (n = 111; P = .0006). In murine models, treatment of transplant donors with human AAT resulted in an increase in interleukin-10 messenger RNA and CD8(+)CD11c(+)CD205(+) major histocompatibility complex class II(+) dendritic cells (DCs), and the prevention or attenuation of acute GVHD in the recipients. Ablation of DCs (in AAT-treated CD11c-DTR donors) decreased CD4(+)CD25(+)FoxP3(+) regulatory T cells to one-third and abrogated the anti-GVHD effect. The graft-versus-leukemia (GVL) effect of donor cells (against A20 tumor cells) was maintained or even enhanced with AAT treatment of the donor, mediated by an expanded population of NK1.1(+), CD49B(+), CD122(+), CD335(+) NKG2D-expressing natural killer (NK) cells. Blockade of NKG2D significantly suppressed the GVL effect. Metabolic analysis showed a high glycolysis-high oxidative phosphorylation profile for NK1.1(+) cells, CD4(+)CD25(+)FoxP3(+) T cells, and CD11c(+) DCs but not for effector T cells, suggesting a cell type-specific effect of AAT. Thus, via altered metabolism, AAT exerts effective GVHD protection while enhancing GVL effects.
© 2014 by The American Society of Hematology.
Figures



Similar articles
-
Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response.Biol Blood Marrow Transplant. 2003 Apr;9(4):243-56. doi: 10.1053/bbmt.2003.50027. Biol Blood Marrow Transplant. 2003. PMID: 12720217
-
The mechanistic study behind suppression of GVHD while retaining GVL activities by myeloid-derived suppressor cells.Leukemia. 2019 Aug;33(8):2078-2089. doi: 10.1038/s41375-019-0394-z. Epub 2019 Feb 8. Leukemia. 2019. PMID: 30737483 Free PMC article.
-
Type I-IFNs control GVHD and GVL responses after transplantation.Blood. 2011 Sep 22;118(12):3399-409. doi: 10.1182/blood-2010-12-325746. Epub 2011 Jun 30. Blood. 2011. PMID: 21719602
-
Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma.Leuk Lymphoma. 2000 Jul;38(3-4):221-34. doi: 10.3109/10428190009087014. Leuk Lymphoma. 2000. PMID: 10830730 Review.
-
Strategies for Enhancing and Preserving Anti-leukemia Effects Without Aggravating Graft-Versus-Host Disease.Front Immunol. 2018 Dec 21;9:3041. doi: 10.3389/fimmu.2018.03041. eCollection 2018. Front Immunol. 2018. PMID: 30619371 Free PMC article. Review.
Cited by
-
A patenting perspective on human neutrophil elastase (HNE) inhibitors (2014-2018) and their therapeutic applications.Expert Opin Ther Pat. 2019 Jul;29(7):555-578. doi: 10.1080/13543776.2019.1630379. Epub 2019 Jun 16. Expert Opin Ther Pat. 2019. PMID: 31204543 Free PMC article. Review.
-
Serpin Family A Member 1 Is Prognostic and Involved in Immunological Regulation in Human Cancers.Int J Mol Sci. 2023 Jul 17;24(14):11566. doi: 10.3390/ijms241411566. Int J Mol Sci. 2023. PMID: 37511325 Free PMC article.
-
Achievement of Tolerance Induction to Prevent Acute Graft-vs.-Host Disease.Front Immunol. 2019 Mar 6;10:309. doi: 10.3389/fimmu.2019.00309. eCollection 2019. Front Immunol. 2019. PMID: 30906290 Free PMC article. Review.
-
Renoprotective Effects of Alpha-1 Antitrypsin against Tacrolimus-Induced Renal Injury.Int J Mol Sci. 2020 Nov 16;21(22):8628. doi: 10.3390/ijms21228628. Int J Mol Sci. 2020. PMID: 33207690 Free PMC article.
-
Clathrin-mediated Endocytosis of Alpha-1 Antitrypsin is Essential for its Protective Function in Islet Cell Survival.Theranostics. 2019 May 31;9(13):3940-3951. doi: 10.7150/thno.31647. eCollection 2019. Theranostics. 2019. PMID: 31281523 Free PMC article.
References
-
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. - PubMed
-
- Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357(15):1472–1475. - PubMed
-
- Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

