Potential roles of noncoding RNAs in environmental epigenetic transgenerational inheritance
- PMID: 25224488
- PMCID: PMC4262681
- DOI: 10.1016/j.mce.2014.09.008
Potential roles of noncoding RNAs in environmental epigenetic transgenerational inheritance
Abstract
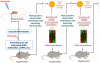
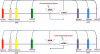
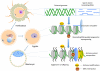
"Epigenetic transgenerational inheritance" (ETI) has been defined as germline (sperm or egg) transmission of epigenetic information between generations in the absence of direct exposures or genetic manipulations. Among reported cases of ETI in mammals, the majority are induced by environmental factors, including environmental toxicants [e.g. agricultural fungicide vinclozolin, plastic additive bisphenol A, pesticide methoxychlor, dioxin, di-(2-ethylhexyl) phthalate, dichlorodiphenyltrichloroethane, and hydrocarbons] and poor nutritional conditions. Although the ETI phenomenon is well established, the underlying mechanism remains elusive. Putative epimutations, including changes in DNA methylation and histone modification patterns, have been reported, but it remains unclear how these epimutations are formed in the first place, and how they are memorized in the germline and then get transmitted to subsequent generations. Based on recent advances in our understanding of regulatory noncoding RNAs (ncRNAs), I propose that ncRNAs are involved in ETI, during both the initial epimutation formation and the subsequent germline transmission of epimutations. ncRNAs can function at epigenetic levels by affecting DNA methylation and histone modifications, thereby changing gene transcriptional activities, which can lead to an altered mRNA transcriptome associated with a disease phenotype. Alternatively, novel or altered ncRNA expression can cause dysregulated post-transcriptional regulation, thus directly affecting the mRNA transcriptome and inducing a disease phenotype. Sperm-borne ncRNAs are potential mediators for epigenetic memory across generations, but they alone may not be sufficient for stable transmission of epimutations across generations. Overall, research on ncRNAs in the context of ETI is urgently needed to shed light on the underlying mechanism of ETI.
Keywords: Disease etiology; Environment; Epigenetics; Gamete; Genetics; Inheritance.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Alleman M, Sidorenko L, McGinnis K, Seshadri V, Dorweiler JE, White J, et al. An RNA-dependent RNA polymerase is required for paramutation in maize. Nature. 2006;442:295–298. - PubMed
-
- Aravin AA, Hannon GJ. Small RNA silencing pathways in germ and stem cells. Cold Spring Harb. Symp. Quant. Biol. 2008;73:283–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

