Local corticotropin releasing hormone (CRH) signals to its receptor CRHR1 during postnatal development of the mouse olfactory bulb
- PMID: 25224546
- PMCID: PMC4362939
- DOI: 10.1007/s00429-014-0888-4
Local corticotropin releasing hormone (CRH) signals to its receptor CRHR1 during postnatal development of the mouse olfactory bulb
Abstract
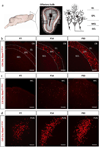
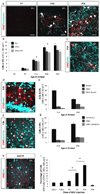
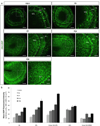
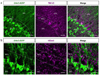
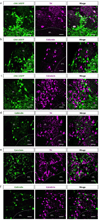
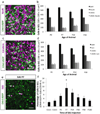
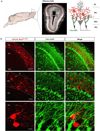
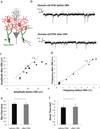
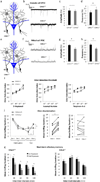
Neuropeptides play important physiological functions during distinct behaviors such as arousal, learning, memory, and reproduction. However, the role of local, extrahypothalamic neuropeptide signaling in shaping synapse formation and neuronal plasticity in the brain is not well understood. Here, we characterize the spatiotemporal expression profile of the neuropeptide corticotropin-releasing hormone (CRH) and its receptor CRHR1 in the mouse OB throughout development. We found that CRH-expressing interneurons are present in the external plexiform layer, that its cognate receptor is expressed by granule cells, and show that both CRH and CRHR1 expression enriches in the postnatal period when olfaction becomes important towards olfactory-related behaviors. Further, we provide electrophysiological evidence that CRHR1-expressing granule cells functionally respond to CRH ligand, and that the physiological circuitry of CRHR1 knockout mice is abnormal, leading to impaired olfactory behaviors. Together, these data suggest a physiologically relevant role for local CRH signaling towards shaping the neuronal circuitry within the mouse OB.
Keywords: CRF; CRFR1; CRH; CRHR1; EPL; GPCR; Granule cell; Neuropeptide; Olfactory.
Figures


Similar articles
-
POU6f1 Mediates Neuropeptide-Dependent Plasticity in the Adult Brain.J Neurosci. 2018 Feb 7;38(6):1443-1461. doi: 10.1523/JNEUROSCI.1641-17.2017. Epub 2018 Jan 5. J Neurosci. 2018. PMID: 29305536 Free PMC article.
-
Reciprocal connectivity between mitral cells and external plexiform layer interneurons in the mouse olfactory bulb.Front Neural Circuits. 2013 Mar 1;7:32. doi: 10.3389/fncir.2013.00032. eCollection 2013. Front Neural Circuits. 2013. PMID: 23459611 Free PMC article.
-
A Subtype of Olfactory Bulb Interneurons Is Required for Odor Detection and Discrimination Behaviors.J Neurosci. 2016 Aug 3;36(31):8210-27. doi: 10.1523/JNEUROSCI.2783-15.2016. J Neurosci. 2016. PMID: 27488640 Free PMC article.
-
Corticotropin releasing hormone receptors: two decades later.Peptides. 2004 Mar;25(3):319-29. doi: 10.1016/j.peptides.2004.02.002. Peptides. 2004. PMID: 15134857 Review.
-
Corticotropin-releasing hormone-binding protein and stress: from invertebrates to humans.Stress. 2017 Sep;20(5):449-464. doi: 10.1080/10253890.2017.1322575. Epub 2017 May 18. Stress. 2017. PMID: 28436309 Free PMC article. Review.
Cited by
-
Organization of the corticotropin-releasing hormone and corticotropin-releasing hormone-binding protein systems in the central nervous system of the sea lamprey Petromyzon marinus.J Comp Neurol. 2023 Jan;531(1):58-88. doi: 10.1002/cne.25412. Epub 2022 Sep 23. J Comp Neurol. 2023. PMID: 36150899 Free PMC article.
-
Fast-spiking interneuron detonation drives high-fidelity inhibition in the olfactory bulb.bioRxiv [Preprint]. 2024 May 8:2024.05.07.592874. doi: 10.1101/2024.05.07.592874. bioRxiv. 2024. Update in: PLoS Biol. 2024 Aug 26;22(8):e3002660. doi: 10.1371/journal.pbio.3002660. PMID: 38766161 Free PMC article. Updated. Preprint.
-
Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: A novel sex difference revealed in the rostral periventricular hypothalamus.Neuroscience. 2017 Oct 11;361:167-178. doi: 10.1016/j.neuroscience.2017.08.016. Epub 2017 Aug 18. Neuroscience. 2017. PMID: 28823817 Free PMC article.
-
Characterization and gonadal hormone regulation of a sexually dimorphic corticotropin-releasing factor receptor 1 cell group.J Comp Neurol. 2019 Apr 15;527(6):1056-1069. doi: 10.1002/cne.24588. Epub 2018 Dec 24. J Comp Neurol. 2019. PMID: 30499109 Free PMC article.
-
Lack of CRH Affects the Behavior but Does Not Affect the Formation of Short-Term Memory.Cell Mol Neurobiol. 2018 Jan;38(1):341-347. doi: 10.1007/s10571-017-0532-y. Epub 2017 Aug 7. Cell Mol Neurobiol. 2018. PMID: 28786031 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

