An interdisciplinary framework for measuring and supporting adherence in HIV prevention trials of ARV-based vaginal rings
- PMID: 25224617
- PMCID: PMC4164000
- DOI: 10.7448/IAS.17.3.19158
An interdisciplinary framework for measuring and supporting adherence in HIV prevention trials of ARV-based vaginal rings
Abstract
Introduction: Product adherence and its measurement have emerged as a critical challenge in the evaluation of new HIV prevention technologies. Long-acting ARV-based vaginal rings may simplify use instructions and require less user behaviour, thereby facilitating adherence. One ARV-based ring is in efficacy trials and others, including multipurpose rings, are in the pipeline. Participant motivations, counselling support and measurement challenges during ring trials must still be addressed. In previous HIV prevention trials, this has been done largely using descriptive and post-hoc methods that are highly variable and minimally evaluated. We outline an interdisciplinary framework for systematically investigating promising strategies to support product uptake and adherence, and to measure adherence in the context of randomized, blinded clinical trials.
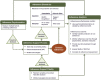
Discussion: The interdisciplinary framework highlights the dual use of adherence measurement (i.e. to provide feedback during trial implementation and to inform interpretation of trial findings) and underscores the complex pathways that connect measurement, adherence support and enacted adherence behaviour. Three inter-related approaches are highlighted: 1) adherence support - sequential efforts to define motivators of study product adherence and to develop, test, refine and evaluate adherence support messages; 2) self-reported psychometric measures - creation of valid and generalizable measures based in easily administered scales that capture vaginal ring use with improved predictive ability at screening, baseline and follow-up that better engage participants in reporting adherence; and 3) more objective measurement of adherence - real-time adherence monitoring and cumulative measurement to correlate adherence with overall product effectiveness through innovative designs, models and prototypes using electronic and biometric technologies to detect ring insertion and/or removal or expulsion. Coordinating research along these three pathways will result in a comprehensive approach to product adherence within clinical trials.
Conclusions: Better measurement of adherence will not, by itself, ensure that future effectiveness trials will be able to address the most basic question: if the product is used per instructions, will it prevent HIV transmission? The challenges to adherence measurement must be addressed as one component of a more integrated system that has as its central focus adherence as a behaviour emerging from the social context of the user.
Keywords: HIV prevention; acceptability; adherence; clinical trial research; measurement; vaginal ring.
Figures
Similar articles
-
Biomarkers and biometric measures of adherence to use of ARV-based vaginal rings.J Int AIDS Soc. 2016 May 2;19(1):20746. doi: 10.7448/IAS.19.1.20746. eCollection 2016. J Int AIDS Soc. 2016. PMID: 27142091 Free PMC article. Review.
-
Women and ARV-based HIV prevention - challenges and opportunities.J Int AIDS Soc. 2014 Sep 8;17(3 Suppl 2):19356. doi: 10.7448/IAS.17.3.19356. eCollection 2014. J Int AIDS Soc. 2014. PMID: 25224621 Free PMC article.
-
Attitudes and perceptions towards novel objective measures of ARV-based vaginal ring use: Results from a global stakeholder survey.PLoS One. 2017 Jul 14;12(7):e0180963. doi: 10.1371/journal.pone.0180963. eCollection 2017. PLoS One. 2017. PMID: 28708847 Free PMC article.
-
Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa.J Int AIDS Soc. 2014 Sep 8;17(3 Suppl 2):19146. doi: 10.7448/IAS.17.3.19146. eCollection 2014. J Int AIDS Soc. 2014. PMID: 25224610 Free PMC article. Clinical Trial.
-
Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice.Curr Opin HIV AIDS. 2016 Jan;11(1):10-7. doi: 10.1097/COH.0000000000000220. Curr Opin HIV AIDS. 2016. PMID: 26633638 Free PMC article. Review.
Cited by
-
Planning ahead for implementation of long-acting HIV prevention: challenges and opportunities.Curr Opin HIV AIDS. 2015 Jul;10(4):290-5. doi: 10.1097/COH.0000000000000159. Curr Opin HIV AIDS. 2015. PMID: 26049956 Free PMC article. Review.
-
Development and clinical assessment of new objective adherence markers for four microbicide delivery systems used in HIV prevention studies.Clin Transl Med. 2018 Nov 7;7(1):37. doi: 10.1186/s40169-018-0213-6. Clin Transl Med. 2018. PMID: 30402770 Free PMC article.
-
Biomarkers and biometric measures of adherence to use of ARV-based vaginal rings.J Int AIDS Soc. 2016 May 2;19(1):20746. doi: 10.7448/IAS.19.1.20746. eCollection 2016. J Int AIDS Soc. 2016. PMID: 27142091 Free PMC article. Review.
-
Women and ARV-based HIV prevention - challenges and opportunities.J Int AIDS Soc. 2014 Sep 8;17(3 Suppl 2):19356. doi: 10.7448/IAS.17.3.19356. eCollection 2014. J Int AIDS Soc. 2014. PMID: 25224621 Free PMC article.
-
Attitudes and perceptions towards novel objective measures of ARV-based vaginal ring use: Results from a global stakeholder survey.PLoS One. 2017 Jul 14;12(7):e0180963. doi: 10.1371/journal.pone.0180963. eCollection 2017. PLoS One. 2017. PMID: 28708847 Free PMC article.
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical