Spectroscopic characterization and mechanistic investigation of P-methyl transfer by a radical SAM enzyme from the marine bacterium Shewanella denitrificans OS217
- PMID: 25224746
- PMCID: PMC4362910
- DOI: 10.1016/j.bbapap.2014.09.009
Spectroscopic characterization and mechanistic investigation of P-methyl transfer by a radical SAM enzyme from the marine bacterium Shewanella denitrificans OS217
Abstract
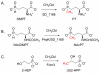
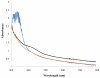
Natural products containing carbon-phosphorus bonds elicit important bioactivity in many organisms. l-Phosphinothricin contains the only known naturally-occurring carbon-phosphorus-carbon bond linkage. In actinomycetes, the cobalamin-dependent radical S-adenosyl-l-methionine (SAM) methyltransferase PhpK catalyzes the formation of the second C-P bond to generate the complete C-P-C linkage in phosphinothricin. Here we use electron paramagnetic resonance and nuclear magnetic resonance spectroscopies to characterize and demonstrate the activity of a cobalamin-dependent radical SAM methyltransferase denoted SD_1168 from Shewanella denitrificans OS217, a marine bacterium that has not been reported to synthesize phosphinothricin. Recombinant, refolded, and reconstituted SD_1168 binds a four-iron, four-sulfur cluster that interacts with SAM and cobalamin. In the presence of SAM, a reductant, and methylcobalamin, SD_1168 surprisingly catalyzes the P-methylation of N-acetyl-demethylphosphinothricin and demethylphosphinothricin to produce N-acetyl-phosphinothricin and phosphinothricin, respectively. In addition, this enzyme is active in the absence of methylcobalamin if the strong reductant titanium (III) citrate and hydroxocobalamin are provided. When incubated with [methyl-(13)C] cobalamin and titanium citrate, both [methyl-(13)C] and unlabeled N-acetylphosphinothricin are produced. Our results suggest that SD_1168 catalyzes P-methylation using radical SAM-dependent chemistry with cobalamin as a coenzyme. In light of recent genomic information, the discovery of this P-methyltransferase suggests that S. denitrificans produces a phosphinate natural product.
Keywords: Cobalamin; Methyltransferase; Phosphinate; Radical S-adenosyl-l-methionine.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
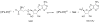

Similar articles
-
In vitro phosphinate methylation by PhpK from Kitasatospora phosalacinea.Biochemistry. 2011 Oct 25;50(42):8986-8. doi: 10.1021/bi201220r. Epub 2011 Sep 28. Biochemistry. 2011. PMID: 21950770 Free PMC article.
-
A Cobalamin-Dependent Radical SAM Enzyme Catalyzes the Unique Cα -Methylation of Glutamine in Methyl-Coenzyme M Reductase.Angew Chem Int Ed Engl. 2022 Aug 8;61(32):e202204198. doi: 10.1002/anie.202204198. Epub 2022 Jun 29. Angew Chem Int Ed Engl. 2022. PMID: 35638156 Free PMC article.
-
Initial characterization of Fom3 from Streptomyces wedmorensis: The methyltransferase in fosfomycin biosynthesis.Arch Biochem Biophys. 2014 Feb 1;543:67-73. doi: 10.1016/j.abb.2013.12.004. Epub 2013 Dec 24. Arch Biochem Biophys. 2014. PMID: 24370735 Free PMC article.
-
Cobalamin-dependent radical S-adenosyl-l-methionine enzymes in natural product biosynthesis.Nat Prod Rep. 2018 Aug 15;35(8):707-720. doi: 10.1039/c7np00059f. Nat Prod Rep. 2018. PMID: 30079906 Review.
-
Radical-mediated enzymatic methylation: a tale of two SAMS.Acc Chem Res. 2012 Apr 17;45(4):555-64. doi: 10.1021/ar200202c. Epub 2011 Nov 18. Acc Chem Res. 2012. PMID: 22097883 Free PMC article. Review.
Cited by
-
Studies of GenK and OxsB, two B12-dependent radical SAM enzymes involved in natural product biosynthesis.Methods Enzymol. 2022;669:71-90. doi: 10.1016/bs.mie.2021.12.014. Epub 2022 Feb 3. Methods Enzymol. 2022. PMID: 35644181 Free PMC article.
-
Analysis of Electrochemical Properties of S-Adenosyl-l-methionine and Implications for Its Role in Radical SAM Enzymes.J Am Chem Soc. 2019 Jul 17;141(28):11019-11026. doi: 10.1021/jacs.9b00933. Epub 2019 Jul 8. J Am Chem Soc. 2019. PMID: 31283208 Free PMC article.
-
Consecutive radical S-adenosylmethionine methylations form the ethyl side chain in thienamycin biosynthesis.Proc Natl Acad Sci U S A. 2015 Aug 18;112(33):10354-8. doi: 10.1073/pnas.1508615112. Epub 2015 Aug 3. Proc Natl Acad Sci U S A. 2015. PMID: 26240322 Free PMC article.
-
Methanogenesis marker protein 10 (Mmp10) from Methanosarcina acetivorans is a radical S-adenosylmethionine methylase that unexpectedly requires cobalamin.J Biol Chem. 2019 Aug 2;294(31):11712-11725. doi: 10.1074/jbc.RA119.007609. Epub 2019 May 20. J Biol Chem. 2019. PMID: 31113866 Free PMC article.
-
Evolution of Methods for the Study of Cobalamin-Dependent Radical SAM Enzymes.ACS Bio Med Chem Au. 2022 Feb 16;2(1):4-10. doi: 10.1021/acsbiomedchemau.1c00032. Epub 2021 Oct 13. ACS Bio Med Chem Au. 2022. PMID: 35341020 Free PMC article.
References
-
- Steinrucken HC, Amrhein N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem. Biophys. Res. Commun. 1980;94:1207–1212. - PubMed
-
- De Clercq E, Holy A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug. Discov. 2005;4:928–940. - PubMed
-
- Leadbetter MR, Adams SM, Bazzini B, Fatheree PR, Karr DE, Krause KM, Lam BM, Linsell MS, Nodwell MB, Pace JL, Quast K, Shaw JP, Soriano E, Trapp SG, Villena JD, Wu TX, Christensen BG, Judice JK. Hydrophobic vancomycin derivatives with improved ADME properties: discovery of telavancin (TD-6424) J. Antibiot. (Tokyo) 2004;57:326–336. - PubMed
-
- Thompson CJ, Seto H. Bialaphos. Biotechnology. 1995;28:197–222. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

