Delayed cutaneous wound closure in HO-2 deficient mice despite normal HO-1 expression
- PMID: 25224969
- PMCID: PMC4302653
- DOI: 10.1111/jcmm.12389
Delayed cutaneous wound closure in HO-2 deficient mice despite normal HO-1 expression
Abstract
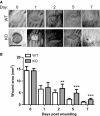
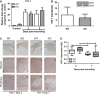
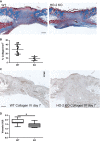
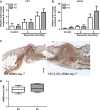
Impaired wound healing can lead to scarring, and aesthetical and functional problems. The cytoprotective haem oxygenase (HO) enzymes degrade haem into iron, biliverdin and carbon monoxide. HO-1 deficient mice suffer from chronic inflammatory stress and delayed cutaneous wound healing, while corneal wound healing in HO-2 deficient mice is impaired with exorbitant inflammation and absence of HO-1 expression. This study addresses the role of HO-2 in cutaneous excisional wound healing using HO-2 knockout (KO) mice. Here, we show that HO-2 deficiency also delays cutaneous wound closure compared to WT controls. In addition, we detected reduced collagen deposition and vessel density in the wounds of HO-2 KO mice compared to WT controls. Surprisingly, wound closure in HO-2 KO mice was accompanied by an inflammatory response comparable to WT mice. HO-1 induction in HO-2 deficient skin was also similar to WT controls and may explain this protection against exaggerated cutaneous inflammation but not the delayed wound closure. Proliferation and myofibroblast differentiation were similar in both two genotypes. Next, we screened for candidate genes to explain the observed delayed wound closure, and detected delayed gene and protein expression profiles of the chemokine (C-X-C) ligand-11 (CXCL-11) in wounds of HO-2 KO mice. Abnormal regulation of CXCL-11 has been linked to delayed wound healing and disturbed angiogenesis. However, whether aberrant CXCL-11 expression in HO-2 KO mice is caused by or is causing delayed wound healing needs to be further investigated.
Keywords: haem oxygenase; skin; wound healing.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Sidgwick GP, Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol. 2012;26:141–52. - PubMed
-
- Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

