The Src family kinases Hck, Fgr, and Lyn are critical for the generation of the in vivo inflammatory environment without a direct role in leukocyte recruitment
- PMID: 25225462
- PMCID: PMC4172222
- DOI: 10.1084/jem.20132496
The Src family kinases Hck, Fgr, and Lyn are critical for the generation of the in vivo inflammatory environment without a direct role in leukocyte recruitment
Abstract
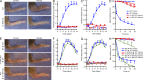
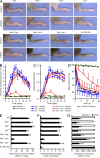
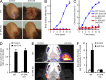
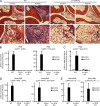
Although Src family kinases participate in leukocyte function in vitro, such as integrin signal transduction, their role in inflammation in vivo is poorly understood. We show that Src family kinases play a critical role in myeloid cell-mediated in vivo inflammatory reactions. Mice lacking the Src family kinases Hck, Fgr, and Lyn in the hematopoietic compartment were completely protected from autoantibody-induced arthritis and skin blistering disease, as well as from the reverse passive Arthus reaction, with functional overlap between the three kinases. Though the overall phenotype resembled the leukocyte recruitment defect observed in β2 integrin-deficient (CD18(-/-)) mice, Hck(-/-)Fgr(-/-)Lyn(-/-) neutrophils and monocytes/macrophages had no cell-autonomous in vivo or in vitro migration defect. Instead, Src family kinases were required for the generation of the inflammatory environment in vivo and for the release of proinflammatory mediators from neutrophils and macrophages in vitro, likely due to their role in Fcγ receptor signal transduction. Our results suggest that infiltrating myeloid cells release proinflammatory chemokine, cytokine, and lipid mediators that attract further neutrophils and monocytes from the circulation in a CD18-dependent manner. Src family kinases are required for the generation of the inflammatory environment but not for the intrinsic migratory ability of myeloid cells.
© 2014 Kovács et al.
Figures
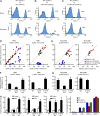

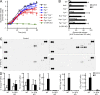
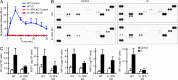
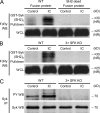

Comment in
-
Fueling the fire: Src family kinases drive inflammation.J Exp Med. 2014 Sep 22;211(10):1922. doi: 10.1084/jem.21110insight1. J Exp Med. 2014. PMID: 25246388 Free PMC article. No abstract available.
Similar articles
-
The Src-Family Kinases Hck and Fgr Regulate Early Lipopolysaccharide-Induced Myeloid Cell Recruitment into the Lung and Their Ability To Secrete Chemokines.J Immunol. 2015 Sep 1;195(5):2383-95. doi: 10.4049/jimmunol.1402011. Epub 2015 Jul 31. J Immunol. 2015. PMID: 26232427 Free PMC article.
-
Myeloid Src kinases regulate phagocytosis and oxidative burst in pneumococcal meningitis by activating NADPH oxidase.J Leukoc Biol. 2008 Oct;84(4):1141-50. doi: 10.1189/jlb.0208118. Epub 2008 Jul 14. J Leukoc Biol. 2008. PMID: 18625913 Free PMC article.
-
Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn.J Exp Med. 1997 May 5;185(9):1661-70. doi: 10.1084/jem.185.9.1661. J Exp Med. 1997. PMID: 9151903 Free PMC article.
-
Integrin signal transduction in myeloid leukocytes.J Leukoc Biol. 1999 Mar;65(3):313-20. doi: 10.1002/jlb.65.3.313. J Leukoc Biol. 1999. PMID: 10080533 Review.
-
The Src-family Kinase Lyn in Immunoreceptor Signaling.Endocrinology. 2021 Oct 1;162(10):bqab152. doi: 10.1210/endocr/bqab152. Endocrinology. 2021. PMID: 34320188 Free PMC article. Review.
Cited by
-
A panel of seven immune-related genes can serve as a good predictive biomarker for cervical squamous cell carcinoma.Front Genet. 2022 Nov 2;13:1024508. doi: 10.3389/fgene.2022.1024508. eCollection 2022. Front Genet. 2022. PMID: 36406134 Free PMC article.
-
Cutaneous kinase activity correlates with treatment outcomes following PI3K delta inhibition in mice with experimental pemphigoid diseases.Front Immunol. 2022 Sep 28;13:865241. doi: 10.3389/fimmu.2022.865241. eCollection 2022. Front Immunol. 2022. PMID: 36248903 Free PMC article.
-
Visualization of autoantibodies and neutrophils in vivo identifies novel checkpoints in autoantibody-induced tissue injury.Sci Rep. 2020 Mar 11;10(1):4509. doi: 10.1038/s41598-020-60233-w. Sci Rep. 2020. PMID: 32161277 Free PMC article.
-
Epiisopiloturine, an Alkaloid from Pilocarpus microphyllus, Attenuates LPS-Induced Neuroinflammation by Interfering in the TLR4/NF-κB-MAPK Signaling Pathway in Microglial Cells.Oxid Med Cell Longev. 2023 Apr 28;2023:4752502. doi: 10.1155/2023/4752502. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 37151606 Free PMC article.
-
Profiles of microRNA networks in intestinal epithelial cells in a mouse model of colitis.Sci Rep. 2015 Dec 9;5:18174. doi: 10.1038/srep18174. Sci Rep. 2015. PMID: 26647826 Free PMC article.
References
-
- Chen, H., Mócsai A., Zhang H., Ding R.X., Morisaki J.H., White M., Rothfork J.M., Heiser P., Colucci-Guyon E., Lowell C.A., et al. . 2003. Role for plastin in host defense distinguishes integrin signaling from cell adhesion and spreading. Immunity. 19:95–104 10.1016/S1074-7613(03)00172-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

